COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08FUR
|
|||
| Drug Name |
IMU-838
|
|||
| Synonyms |
Vidofludimus; 717824-30-1; 4SC-101; SC12267; UNII-8Y1PJ3VG81; SC 12267; CHEMBL197194; 8Y1PJ3VG81; Vidofludimus(4SC-101; SC12267); Vidofludimus [INN]; SCHEMBL247888; GTPL9860; KS-00000TTT; BDBM16111; EX-A546; IMU-838; DTXSID50431325; HMS3740I15; AOB87354; BCP14555; ZINC14960644; s7262; AK; Vidofludimus
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 2/3 | [1] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
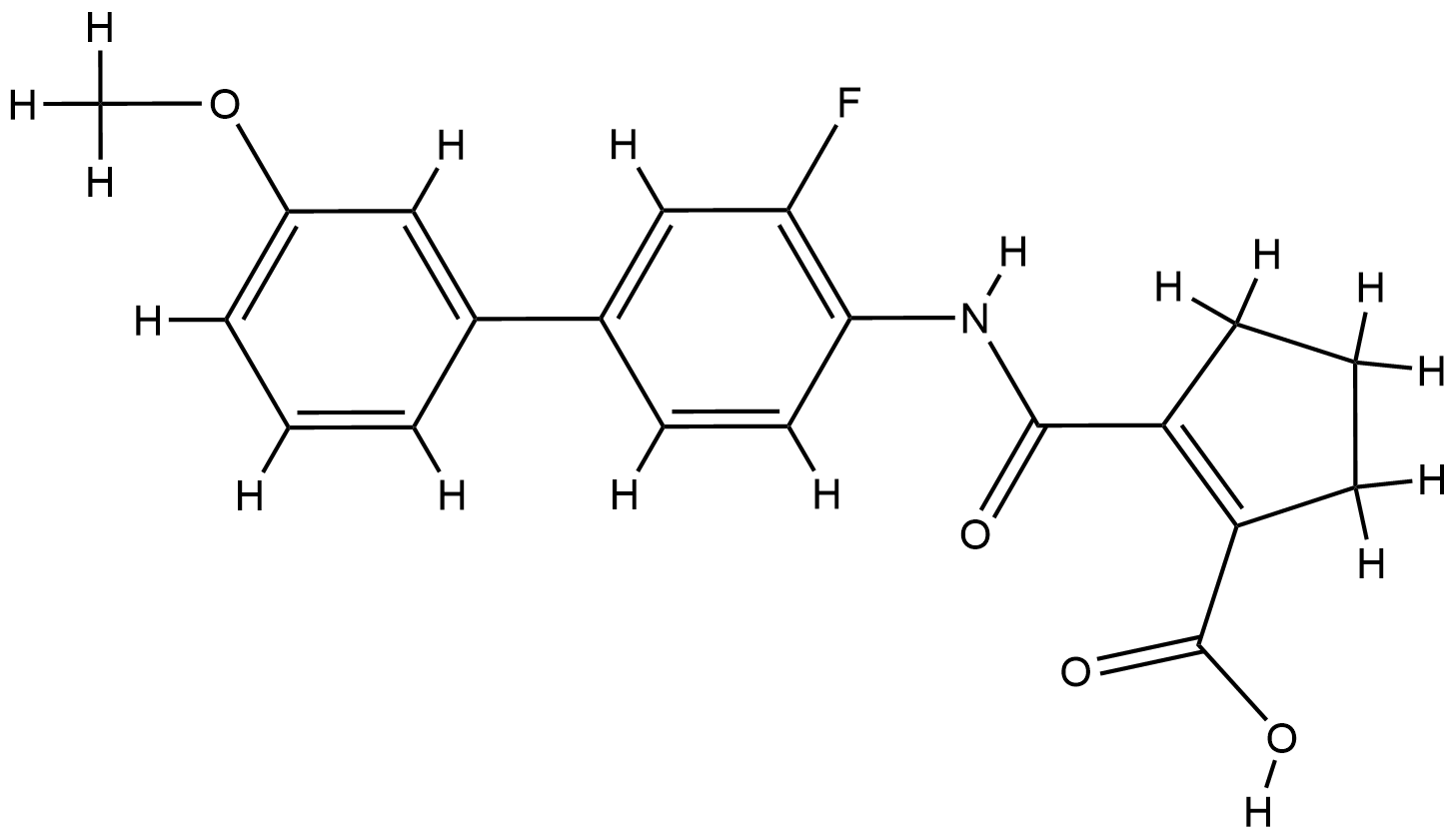 |
Download2D MOL |
||
| Formula |
C20H18FNO4
|
|||
| Canonical SMILES |
COC1=CC=CC(=C1)C2=CC(=C(C=C2)NC(=O)C3=C(CCC3)C(=O)O)F
|
|||
| InChI |
1S/C20H18FNO4/c1-26-14-5-2-4-12(10-14)13-8-9-18(17(21)11-13)22-19(23)15-6-3-7-16(15)20(24)25/h2,4-5,8-11H,3,6-7H2,1H3,(H,22,23)(H,24,25)
|
|||
| InChIKey |
XPRDUGXOWVXZLL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 717824-30-1
|
|||
| PubChem Compound ID | ||||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN dihydroorotate dehydrogenase (DHODH) | Target Info | Inhibitor | [2] |
| IMU-838 is a small molecule dihydroorotate dehydrogenase (DHODH) inhibitor, a key enzyme of pyrimidine de novo biosynthesis. Inhibiton of DHODH by IMU-838 suppresses metabolically activate T and B immune cells experience metabolic stress, and the release of Th1 and Th17 key cytokines including IL-17A, IL-17F and IFNg, thereby reducing inflammation in COVID-19. IMU-838 has successfully demonstrated preclinical activity against SARS-CoV-2. Specifically, IMU-838 was observed to inhibit replication of clinical isolates of SARS-CoV-2 associated with COVID-19. | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04379271) A Study to Evaluate the Efficacy, Safety and Tolerability of IMU-838 as Addition to Investigator's Choice of Standard of Care Therapy, in Patients With Coronavirus Disease 19 (COVID-19). U.S. National Institutes of Health. | |||
| 2 | IMU-838 Targeting DHODH | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

