COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V6GN
|
|||
| Drug Name |
Chlorpromazine
|
|||
| Synonyms |
Aminasine; Aminazin; Aminazine; Ampliactil; Amplicitil; Amplictil; BC 135; Chlor-PZ; Chlor-Promanyl; Chlordelazine; Chlorderazin; Chloropromazine; Chlorpromados; Chlorpromanyl (discontinued); Chlorpromazin; Chlorpromazine (USP/INN); Chlorpromazine Tannate; Chlorpromazine [USAN:INN:BAN]; Chlorpromazinum; Chlorpromazinum [INN-Latin]; Clorpromazina; Clorpromazina [INN-Spanish]; Clorpromazina [Italian]; Contomin; Cromedazine; Elmarin; Esmind; Fenactil; Fenaktyl; Fraction AB; HL 5746; JHICC02042; Largactil; Largactil (TN); Largactil Liquid; Largactil Oral Drops; Largactilothiazine; Largactyl; Megaphen; Novo-Chlorpromazine; Novomazina; Phenactyl; Phenathyl; Phenothiazine hydrochloride; Plegomasine; Plegomazin; Prazilpromactil; Proma; Promactil; Promazil; Propaphen; Propaphenin; Prozil; Psychozine; SKF 2601-A; SKF 2601A; SKF-2601; Sanopron; Thorazine; Thorazine (TN); Thorazine Spansule; Thorazine Suppositories; Thorazine hydrochloride; Torazina; Wintermin; Z80
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 3 | [1] | Middle East Respiratory Syndrome (MERS) | Investigative | [2] | Severe acute respiratory syndrome (SARS) | Investigative | [2] |
| Other Indication | Schizophrenia | Withdrawn from market | [3] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
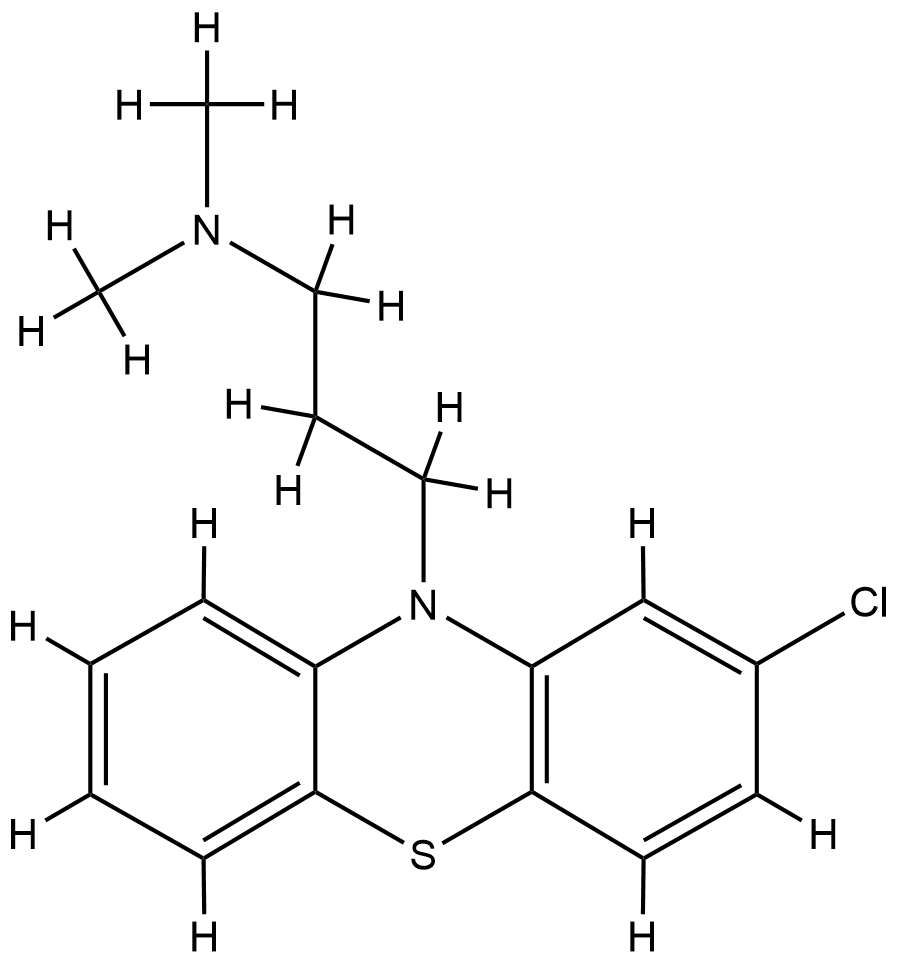 |
Download2D MOL |
||
| Formula |
C17H19ClN2S
|
|||
| Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl
|
|||
| InChI |
1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3
|
|||
| InChIKey |
ZPEIMTDSQAKGNT-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 34468-21-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9123, 441206, 602724, 841999, 4332427, 7847336, 7978926, 8149253, 8151764, 10524433, 10588932, 11110990, 11110991, 11120337, 11120825, 11121313, 11335799, 11361038, 11363036, 11364725, 11365598, 11367287, 11368160, 11369849, 11371331, 11372890, 11373945, 11375449, 11376322, 11378013, 11462010, 11466092, 11467212, 11484847, 11485904, 11488972, 11490162, 11492098, 11493956, 12014508, 14923746, 24398080, 25688547, 26611657, 26680205, 26747094, 26747095, 26751620, 26751621, 29221882
|
|||
| ChEBI ID |
CHEBI:3647
|
|||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN clathrin-mediated endocytosis (RME) | Target Info | Inhibitor | [2], [4] |
| Chlorpromazine,an inhibitor for clathrin-dependent endocytosis, possess significant inhibitory effect on the entry of SARS CoV-2. Chlorpromazine is active against numerous CoVs, including SARS-CoV and MERS-CoV by affecting the assembly of clathrin-coated pits at the plasma membrane and targeting the endocytosis of CoV during cell entry. | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04366739) Repurposing of Chlorpromazine in Covid-19 Treatment. U.S. National Institutes of Health. | |||
| 2 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||
| 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| 4 | Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19. Int J Biol Sci. 2020 Mar 15;16(10):1724-1731. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

