Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01YVT
|
|||
| Former ID |
DIB016243
|
|||
| Drug Name |
Colesevelam
|
|||
| Synonyms |
CholestaGel; Welchol; Colesevelam hydrochloride; Welchol DM; GT31-104; GT31-104HB
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypercholesterolaemia [ICD-11: 5C80.0] | Approved | [1], [2] | |
| Company |
GelTex Pharmaceuticals Inc
|
|||
| Structure |
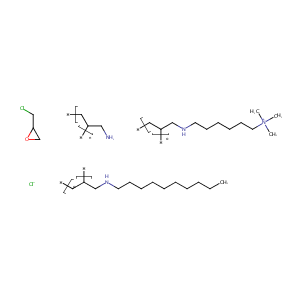 |
Download2D MOL |
||
| Formula |
C31H67Cl3N4O
|
|||
| Canonical SMILES |
CCCCCCCCCCNCC=C.C[N+](C)(C)CCCCCCNCC=C.C=CCN.C1C(O1)CCl.Cl.[Cl-]
|
|||
| InChI |
1S/C13H27N.C12H27N2.C3H5ClO.C3H7N.2ClH/c1-3-5-6-7-8-9-10-11-13-14-12-4-2;1-5-10-13-11-8-6-7-9-12-14(2,3)4;4-1-3-2-5-3;1-2-3-4;;/h4,14H,2-3,5-13H2,1H3;5,13H,1,6-12H2,2-4H3;3H,1-2H2;2H,1,3-4H2;2*1H/q;+1;;;;/p-1
|
|||
| InChIKey |
VTAKZNRDSPNOAU-UHFFFAOYSA-M
|
|||
| CAS Number |
CAS 182815-44-7
|
|||
| PubChem Compound ID | ||||
| ADReCS Drug ID | BADD_D00519 ; BADD_D02431 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | ClinicalTrials.gov (NCT01258075) Colesevelam Pediatric Type 2 Diabetes Mellitus Study. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

