Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07ACT
|
|||
| Former ID |
DAP000145
|
|||
| Drug Name |
Ceftriaxone
|
|||
| Synonyms |
Biotrakson; CTRX; Cefatriaxone; Ceftriaxon; Ceftriaxona; Ceftriaxonum; Ceftriazone; Longacef; Longaceph; Rocefin; Rocephin; Rocephine; CEFTRIAXONE SODIUM; Ceftriaxone intravenous; Ro 139904; Ceftriaxona [INN-Spanish]; Ceftriaxone (INN); Ceftriaxone (TN); Ceftriaxone [USAN:JAN]; Ceftriaxone, Disodium Salt; Ceftriaxonum [INN-Latin]; DRG-0071; Ro13-9904; Rocephin (TN); Ceftriaxone, Disodium Salt, Hemiheptahydrate; Ro-13-9904; (6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-8-oxo-3-(((1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as-triazin-3-yl)thio)methyl)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7(sup 2)-(Z)-(O-methyloxime), sesquaterhydrate; (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-3-{[(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(2-methyl-5,6-dioxo-1H-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-3,4-didehydrocepham-4-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bacterial infection [ICD-11: 1A00-1C4Z; ICD-10: A00-B99] | Approved | [1], [2] | |
| Therapeutic Class |
Antibiotics
|
|||
| Company |
Hoffmann-La Roche pharmaceutical company
|
|||
| Structure |
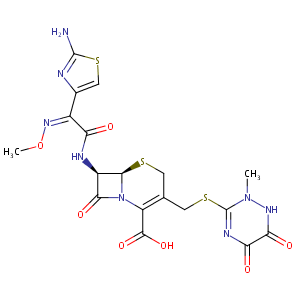 |
Download2D MOL |
||
| Formula |
C18H18N8O7S3
|
|||
| Canonical SMILES |
CN1C(=NC(=O)C(=O)N1)SCC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O
|
|||
| InChI |
1S/C18H18N8O7S3/c1-25-18(22-12(28)13(29)23-25)36-4-6-3-34-15-9(14(30)26(15)10(6)16(31)32)21-11(27)8(24-33-2)7-5-35-17(19)20-7/h5,9,15H,3-4H2,1-2H3,(H2,19,20)(H,21,27)(H,23,29)(H,31,32)/b24-8-/t9-,15-/m1/s1
|
|||
| InChIKey |
VAAUVRVFOQPIGI-SPQHTLEESA-N
|
|||
| CAS Number |
CAS 73384-59-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8908, 602917, 7978887, 11693489, 14886134, 14935103, 15968575, 39470230, 46506458, 48415733, 50050959, 51091963, 57363926, 79861798, 87322639, 92711237, 103240795, 103980019, 113985965, 124766008, 126631063, 126657477, 134224100, 134337467, 134338568, 135011808, 137019029, 140116145, 141914537, 152100037, 160964545, 162178862, 175265409, 176267075, 179151046, 179323748, 196109964, 210279827, 210282150, 223653569, 223680127, 223680436, 226412100, 241132770, 250133954, 252122133
|
|||
| ChEBI ID |
CHEBI:29007
|
|||
| ADReCS Drug ID | BADD_D00405 ; BADD_D00406 | |||
| SuperDrug ATC ID |
J01DD04
|
|||
| SuperDrug CAS ID |
cas=073384595
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacillales | ||||
|
Studied Microbe: Bacillus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bacillus was increased by Ceftriaxone. | |||
|
Studied Microbe: Staphylococcus
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Staphylococcus was decreased by Ceftriaxone. | |||
|
Studied Microbe: Staphylococcus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Staphylococcus was increased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bacteroides was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bifidobacteriales | ||||
|
Studied Microbe: Bifidobacterium
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Bifidobacterium was decreased by Ceftriaxone. | |||
|
Studied Microbe: Bifidobacterium
Show/Hide Hierarchy
|
[6] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Bifidobacterium was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Enterobacterales | ||||
|
Studied Microbe: Enterobacteria
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Enterobacteria was decreased by Ceftriaxone. | |||
|
Studied Microbe: Escherichia
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Escherichia was decreased by Ceftriaxone. | |||
|
Studied Microbe: Escherichia coli
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Escherichia coli was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Clostridium
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Clostridium was decreased by Ceftriaxone. | |||
|
Studied Microbe: Clostridium
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | No significant change | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Clostridium was not significantly changed by Ceftriaxone. | |||
|
Studied Microbe: Clostridium difficile
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Clostridium difficile was increased by Ceftriaxone. | |||
|
Studied Microbe: Eubacterium
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Eubacterium was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Fusobacteriales | ||||
|
Studied Microbe: Fusobacterium
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Fusobacterium was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Enterococcus
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Enterococcus was increased by Ceftriaxone. | |||
|
Studied Microbe: Enterococcus
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Enterococcus was increased by Ceftriaxone. | |||
|
Studied Microbe: Enterococcus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Enterococcus was increased by Ceftriaxone. | |||
|
Studied Microbe: Enterococcus
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Enterococcus was increased by Ceftriaxone. | |||
|
Studied Microbe: Lactobacillus
Show/Hide Hierarchy
|
[6] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Lactobacillus was decreased by Ceftriaxone. | |||
|
Studied Microbe: Lactobacillus
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Lactobacillus was decreased by Ceftriaxone. | |||
|
Studied Microbe: Streptococcus
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Acute bacterial infection | |||
| Description | The abundance of Streptococcus was decreased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Saccharomycetales | ||||
|
Studied Microbe: Candida
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Community acquired pneumonia | |||
| Description | The abundance of Candida was increased by Ceftriaxone. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
|
Studied Microbe: Clostridia
Show/Hide Hierarchy
|
[6] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Healthy | |||
| Description | The abundance of Clostridia was decreased by Ceftriaxone. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Penicillin binding protein (Bact PBP) | Target Info | Modulator | [8] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5326). | |||
| REF 2 | How many modes of action should an antibiotic have Curr Opin Pharmacol. 2008 Oct;8(5):564-73. | |||
| REF 3 | Interindividual variability in biliary excretion of ceftriaxone: effects on biliary lipid metabolism and on intestinal microflora. Eur J Clin Invest. 1988 Jun;18(3):261-6. | |||
| REF 4 | Ceftriaxone: pharmacokinetics and effect on the intestinal microflora in patients with acute bacterial infections. Scand J Infect Dis. 1985;17(1):77-82. | |||
| REF 5 | Effect of step-down therapy of ceftriaxone plus loracarbef versus parenteral therapy of ceftriaxone on the intestinal microflora in patients with community-acquired pneumonia. Clin Microbiol Infect. 2001 Jul;7(7):376-9. | |||
| REF 6 | Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob Agents Chemother. 2004 Oct;48(10):3765-72. | |||
| REF 7 | The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J Antimicrob Chemother. 1985 Mar;15(3):319-26. | |||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

