Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T5DP
|
|||
| Former ID |
DCL000473
|
|||
| Drug Name |
Selumetinib
|
|||
| Synonyms |
Selumetinib; AZD-6244; ARRY142886; AZD6244; ARRY142886; AZD 6244; AZD-6244; 6UH91I579U; ARRY 142886; ARRY-142886; AZD6244 (Selumetinib); AZD6244(Selumetinib); CHEBI:90227; CHEMBL1614701; MEK inhibitors; Selumetinib (AZD6244); UNII-6UH91I579U
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 3 | [1] | |
| Thyroid cancer [ICD-11: 2D10; ICD-10: C73] | Phase 3 | [2] | ||
| Non-small-cell lung cancer [ICD-11: 2C25.Y] | Phase 2 | [3], [4] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Investigative | [5] | ||
| Company |
AstraZeneca
|
|||
| Structure |
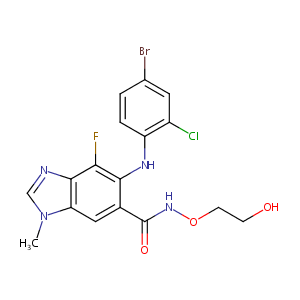 |
Download2D MOL |
||
| Formula |
C17H15BrClFN4O3
|
|||
| Canonical SMILES |
CN1C=NC2=C1C=C(C(=C2F)NC3=C(C=C(C=C3)Br)Cl)C(=O)NOCCO
|
|||
| InChI |
1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26)
|
|||
| InChIKey |
CYOHGALHFOKKQC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 606143-52-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
15117183, 22530930, 39966063, 53837732, 57374224, 76853338, 91146061, 99431772, 123051124, 124490406, 124756930, 124899407, 124963590, 125163737, 125329930, 125569760, 126580498, 126644899, 126665950, 126737693, 131465101, 131477688, 135262198, 135344948, 135602608, 135685252, 135685253, 135685272, 135686160, 135686161, 135686182, 135686183, 135727389, 136368075, 136920282, 136959521, 137220034, 139721905, 143499011, 144115657, 144206913, 152043674, 152235945, 152258818, 152344235, 160647668, 160813024, 162011399, 162037381, 162200063
|
|||
| ChEBI ID |
CHEBI:90227
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01933932) Assess Efficacy & Safety of Selumetinib in Combination With Docetaxel in Patients Receiving 2nd Line Treatment for v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Positive NSCLC (SELECT-1) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5665). | |||
| REF 4 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1479). | |||
| REF 6 | Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells.Br J Cancer.2012 May 8;106(10):1648-59. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

