Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0XQ5X
|
|||
| Drug Name |
Halaven
|
|||
| Synonyms |
Eribulin mesylate; Eribulin (mesylate); Eribulin mesilate; UNII-AV9U0660CW; Eribulin mesylate [USAN]; 441045-17-6; AV9U0660CW; CHEBI:70710; E 7389; E7389; Eribulin mesylate (USAN); B-1939; NSC-707389; Eribulin mesilate (JAN); CHEMBL1683544; QAMYWGZHLCQOOJ-WRNBYXCMSA-N; HY-13442A; AKOS030238218; CS-2803; D08914; 2-(3-amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9H,15H-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopent
Click to Show/Hide
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65; ICD-10: C50, C79.51] | Approved | [1] | |
| Bladder cancer [ICD-11: 2C94; ICD-9: 188] | Phase 1/2 | [2] | ||
| Company |
Eisai Woodcliff Lake, NJ
|
|||
| Structure |
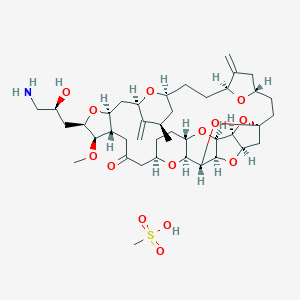 |
Download2D MOL |
||
| Formula |
C41H63NO14S
|
|||
| Canonical SMILES |
CC1CC2CCC3C(=C)CC(O3)CCC45CC6C(O4)C7C(O6)C(O5)C8C(O7)CCC(O8)CC(=O)CC9C(CC(C1=C)O2)OC(C9OC)CC(CN)O.CS(=O)(=O)O
|
|||
| InChI |
1S/C40H59NO11.CH4O3S/c1-19-11-24-5-7-28-20(2)12-26(45-28)9-10-40-17-33-36(51-40)37-38(50-33)39(52-40)35-29(49-37)8-6-25(47-35)13-22(42)14-27-31(16-30(46-24)21(19)3)48-32(34(27)44-4)15-23(43)18-41;1-5(2,3)4/h19,23-39,43H,2-3,5-18,41H2,1,4H3;1H3,(H,2,3,4)/t19-,23+,24+,25-,26+,27+,28+,29+,30-,31+,32-,33-,34-,35+,36+,37+,38-,39+,40+;/m1./s1
|
|||
| InChIKey |
QAMYWGZHLCQOOJ-WRNBYXCMSA-N
|
|||
| CAS Number |
CAS 441045-17-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:70710
|
|||
| ADReCS Drug ID | BADD_D00798 ; BADD_D00799 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2010 | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

