Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T00140
(Former ID: TTDS00112)
|
|||||
| Target Name |
Arachidonate 5-lipoxygenase (5-LOX)
|
|||||
| Synonyms |
LOG5; 5-lipoxygenase; 5-LO
Click to Show/Hide
|
|||||
| Gene Name |
ALOX5
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Asthma [ICD-11: CA23] | |||||
| 2 | Filariasis [ICD-11: 1F66] | |||||
| 3 | Thrombocytopenia [ICD-11: 3B64] | |||||
| Function |
Catalyzes the first step in leukotriene biosynthesis, and thereby plays a role in inflammatory processes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Oxygenase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.13.11.34
|
|||||
| Sequence |
MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY YYLSPDRIPNSVAI Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A03634 | |||||
| HIT2.0 ID | T56TXY | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 3 Approved Drugs | + | ||||
| 1 | Diethylcarbamazine | Drug Info | Approved | Lymphatic filariasis | [2], [3] | |
| 2 | Zileuton | Drug Info | Approved | Asthma | [4], [5] | |
| 3 | 3,4-Dihydroxycinnamic Acid | Drug Info | Phase 4 | Thrombocytopenia | [6] | |
| Clinical Trial Drug(s) | [+] 22 Clinical Trial Drugs | + | ||||
| 1 | ABT-761 | Drug Info | Phase 3 | Asthma | [7] | |
| 2 | Avastin+/-Tarceva | Drug Info | Phase 3 | Non-small-cell lung cancer | [8] | |
| 3 | Flobufen | Drug Info | Phase 3 | Rheumatoid arthritis | [9] | |
| 4 | FPL-62064 | Drug Info | Phase 3 | Inflammation | [10] | |
| 5 | Tenidap | Drug Info | Phase 3 | Rheumatoid arthritis | [11], [12] | |
| 6 | Darbufelone | Drug Info | Phase 2/3 | Asthma | [13] | |
| 7 | BAICALEIN | Drug Info | Phase 2 | Influenza virus infection | [14] | |
| 8 | BIM23A760 | Drug Info | Phase 2 | Acromegaly | [15], [16] | |
| 9 | CMI-392 | Drug Info | Phase 2 | Psoriasis vulgaris | [17] | |
| 10 | E-6700 | Drug Info | Phase 2 | Asthma | [18] | |
| 11 | MK-866 | Drug Info | Phase 2 | Discovery agent | [19] | |
| 12 | PF-4191834 | Drug Info | Phase 2 | Asthma | [20] | |
| 13 | PTC299 | Drug Info | Phase 2 | Rheumatoid arthritis | [21], [22] | |
| 14 | Q301 | Drug Info | Phase 2 | Atopic dermatitis | [23] | |
| 15 | Rilopirox | Drug Info | Phase 2 | Fungal infection | [24] | |
| 16 | TA-270 | Drug Info | Phase 2 | Asthma | [25] | |
| 17 | Tepoxalin | Drug Info | Phase 2 | Asthma | [26] | |
| 18 | Tipelukast | Drug Info | Phase 2 | Asthma | [27] | |
| 19 | UCB-35440 | Drug Info | Phase 2 | Rhinitis | [28] | |
| 20 | WY-50295-tromethamine | Drug Info | Phase 2 | Asthma | [29] | |
| 21 | BF-389 | Drug Info | Phase 1 | Rheumatoid arthritis | [30] | |
| 22 | SKF-105809 | Drug Info | Phase 1 | Pain | [31] | |
| Discontinued Drug(s) | [+] 43 Discontinued Drugs | + | ||||
| 1 | Ibuproxam | Drug Info | Withdrawn from market | Respiratory disease | [32] | |
| 2 | CJ-13610 | Drug Info | Discontinued in Phase 2 | Asthma | [33], [34] | |
| 3 | DuP-654 | Drug Info | Discontinued in Phase 2 | Pruritus | [35] | |
| 4 | E-3040 | Drug Info | Discontinued in Phase 2 | Thrombosis | [36] | |
| 5 | E-6080 | Drug Info | Discontinued in Phase 2 | Asthma | [37] | |
| 6 | ETH615 | Drug Info | Discontinued in Phase 2 | Dermatitis | [38] | |
| 7 | FPL-64170 | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [39] | |
| 8 | Linetastine | Drug Info | Discontinued in Phase 2 | Rhinitis | [40] | |
| 9 | MK-591 | Drug Info | Discontinued in Phase 2 | Asthma | [41] | |
| 10 | MK-886 | Drug Info | Discontinued in Phase 2 | Asthma | [42], [43] | |
| 11 | MLN-977 | Drug Info | Discontinued in Phase 2 | Chronic obstructive pulmonary disease | [44] | |
| 12 | OPC-21268 | Drug Info | Discontinued in Phase 2 | Cardiac disease | [45], [46] | |
| 13 | R-68151 | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [47] | |
| 14 | SC-45662 | Drug Info | Discontinued in Phase 2 | Asthma | [48] | |
| 15 | TEBUFELONE | Drug Info | Discontinued in Phase 2 | Pain | [49] | |
| 16 | AZD-4407 | Drug Info | Discontinued in Phase 1 | Chronic obstructive pulmonary disease | [50] | |
| 17 | CD-581 | Drug Info | Discontinued in Phase 1 | Atopic dermatitis | [51] | |
| 18 | Licofelone | Drug Info | Discontinued in Phase 1 | Osteoarthritis | [52] | |
| 19 | A-78773 | Drug Info | Terminated | Asthma | [53] | |
| 20 | A-79175 | Drug Info | Terminated | Asthma | [54] | |
| 21 | A-80263 | Drug Info | Terminated | Inflammation | [55] | |
| 22 | AA-861 | Drug Info | Terminated | Allergy | [56] | |
| 23 | BI-L-357 | Drug Info | Terminated | Asthma | [57] | |
| 24 | BU-4601A | Drug Info | Terminated | Asthma | [58] | |
| 25 | BW A4C | Drug Info | Terminated | Arthritis | [59] | |
| 26 | BW B70C | Drug Info | Terminated | Asthma | [60] | |
| 27 | BW755C | Drug Info | Terminated | Inflammation | [61] | |
| 28 | CGS-26529 | Drug Info | Terminated | Inflammation | [62] | |
| 29 | CI-986 | Drug Info | Terminated | Rheumatoid arthritis | [63] | |
| 30 | CMI-206 | Drug Info | Terminated | Inflammation | [64] | |
| 31 | Epocarbazolin-A | Drug Info | Terminated | Asthma | [65] | |
| 32 | ER-34122 | Drug Info | Terminated | Inflammation | [66], [67] | |
| 33 | KC-11404 | Drug Info | Terminated | Asthma | [68] | |
| 34 | KC-11425 | Drug Info | Terminated | Asthma | [69] | |
| 35 | LY-221068 | Drug Info | Terminated | Arthritis | [70] | |
| 36 | PD-146176 | Drug Info | Terminated | Arteriosclerosis | [71] | |
| 37 | R zileuton | Drug Info | Terminated | Asthma | [72] | |
| 38 | RWJ-63556 | Drug Info | Terminated | Arthritis | [73] | |
| 39 | Sch-40120 | Drug Info | Terminated | Pruritus | [74] | |
| 40 | SKF-104351 | Drug Info | Terminated | Rheumatoid arthritis | [75] | |
| 41 | WY-28342 | Drug Info | Terminated | Rheumatoid arthritis | [76] | |
| 42 | ZD-7717 | Drug Info | Terminated | Asthma | [77] | |
| 43 | ZM-230487 | Drug Info | Terminated | Asthma | [78] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 160 Inhibitor drugs | + | ||||
| 1 | Diethylcarbamazine | Drug Info | [1] | |||
| 2 | Zileuton | Drug Info | [79], [80], [81], [82], [83], [84] | |||
| 3 | 3,4-Dihydroxycinnamic Acid | Drug Info | [85], [86], [87] | |||
| 4 | Silymarin | Drug Info | [88] | |||
| 5 | ABT-761 | Drug Info | [89], [90], [91] | |||
| 6 | Avastin+/-Tarceva | Drug Info | [92] | |||
| 7 | BAICALEIN | Drug Info | [93] | |||
| 8 | BIM23A760 | Drug Info | [84], [94] | |||
| 9 | PF-4191834 | Drug Info | [95] | |||
| 10 | PTC299 | Drug Info | [96] | |||
| 11 | Q301 | Drug Info | [97] | |||
| 12 | Rilopirox | Drug Info | [98] | |||
| 13 | TA-270 | Drug Info | [99] | |||
| 14 | Tepoxalin | Drug Info | [100] | |||
| 15 | WY-50295-tromethamine | Drug Info | [29] | |||
| 16 | SKF-105809 | Drug Info | [31] | |||
| 17 | Ibuproxam | Drug Info | [103] | |||
| 18 | CJ-13610 | Drug Info | [34] | |||
| 19 | CV-6504 | Drug Info | [104], [105] | |||
| 20 | DuP-654 | Drug Info | [106] | |||
| 21 | E-3040 | Drug Info | [107] | |||
| 22 | E-6080 | Drug Info | [108] | |||
| 23 | ETH615 | Drug Info | [84] | |||
| 24 | FPL-64170 | Drug Info | [109] | |||
| 25 | Linetastine | Drug Info | [84], [110] | |||
| 26 | MK-591 | Drug Info | [111], [112] | |||
| 27 | MLN-977 | Drug Info | [114] | |||
| 28 | OPC-21268 | Drug Info | [115] | |||
| 29 | R-68151 | Drug Info | [84], [116] | |||
| 30 | SC-45662 | Drug Info | [117] | |||
| 31 | TEBUFELONE | Drug Info | [118] | |||
| 32 | AZD-4407 | Drug Info | [119] | |||
| 33 | CD-581 | Drug Info | [2] | |||
| 34 | Licofelone | Drug Info | [120], [121], [122], [123] | |||
| 35 | A-78773 | Drug Info | [124] | |||
| 36 | A-79175 | Drug Info | [125], [126] | |||
| 37 | A-80263 | Drug Info | [127] | |||
| 38 | AA-861 | Drug Info | [128], [129], [130], [131] | |||
| 39 | BI-L-357 | Drug Info | [132] | |||
| 40 | BU-4601A | Drug Info | [133] | |||
| 41 | BW A4C | Drug Info | [134], [135], [136] | |||
| 42 | BW B70C | Drug Info | [136] | |||
| 43 | BW755C | Drug Info | [137] | |||
| 44 | CGS 8515 | Drug Info | [138] | |||
| 45 | CGS-26529 | Drug Info | [139] | |||
| 46 | Epocarbazolin-A | Drug Info | [65] | |||
| 47 | LY-221068 | Drug Info | [142] | |||
| 48 | NAFAZATROM | Drug Info | [143] | |||
| 49 | R zileuton | Drug Info | [145] | |||
| 50 | R-85355 | Drug Info | [116] | |||
| 51 | REV-5901 | Drug Info | [93] | |||
| 52 | ZM-230487 | Drug Info | [150] | |||
| 53 | 1,2-Dihydro-indazol-3-one | Drug Info | [93] | |||
| 54 | 1,2-Dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 55 | 1,3,8-Trihydroxy-6-methyl-10H-anthracen-9-one | Drug Info | [92] | |||
| 56 | 1,5-Dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 57 | 1,8,9-Trimethoxy-9,10-dihydro-anthracene | Drug Info | [92] | |||
| 58 | 1,8-Dichloro-10H-anthracen-9-one | Drug Info | [92] | |||
| 59 | 1,8-Dihydroxy-2-propionyl-10H-anthracen-9-one | Drug Info | [92] | |||
| 60 | 1-Benzyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 61 | 1-furan-2-yl-3-pyridin-2-yl-propenone (FPP-3) | Drug Info | [151] | |||
| 62 | 1-Hydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 63 | 1-Hydroxy-8-methoxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 64 | 1-Methyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 65 | 10-Acetyl-1,8-dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 66 | 10-Benzoyl-1,8-dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 67 | 15-hydroxyeicosatetraenoic acid | Drug Info | [84], [152] | |||
| 68 | 2'-Nitro-biphenyl-4-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 69 | 2-(1H-Indol-3-ylmethyl)-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 70 | 2-(3-Phenyl-propyl)-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 71 | 2-(4-Butoxy-phenoxy)-N-hydroxy-acetamide | Drug Info | [153] | |||
| 72 | 2-(4-Butoxy-phenoxy)-N-hydroxy-N-methyl-acetamide | Drug Info | [153] | |||
| 73 | 2-(4-Butoxy-phenoxy)-N-hydroxy-propionamide | Drug Info | [153] | |||
| 74 | 2-(4-Butoxy-phenyl)-N-hydroxy-N-methyl-acetamide | Drug Info | [153] | |||
| 75 | 2-(4-hydroxylphenyl)-3-(3,5-dihydroxylphenyl) propenoic acid (NNU-hdpa) | Drug Info | [154] | |||
| 76 | 2-(4-Methoxy-phenyl)-5-phenyl-thiazol-4-ol | Drug Info | [155] | |||
| 77 | 2-(4-Phenyl-butyl)-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 78 | 2-Benzyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 79 | 2-Biphenyl-4-yl-N-hydroxy-N-methyl-acetamide | Drug Info | [153] | |||
| 80 | 2-Furan-2-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 81 | 2-Methyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 82 | 2-Naphthalen-1-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 83 | 2-Naphthalen-2-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 84 | 2-Phenethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 85 | 2-Phenyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 86 | 2-Pyridin-2-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 87 | 2-Pyridin-3-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [135], [93] | |||
| 88 | 2-Pyridin-4-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 89 | 2-Thiazol-5-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 90 | 2-Thiophen-2-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [93] | |||
| 91 | 3,4-Dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 92 | 3-(4-Butoxy-phenyl)-N-hydroxy-N-methyl-acrylamide | Drug Info | [153] | |||
| 93 | 3-Benzoyl-N-hydroxy-benzamide | Drug Info | [153] | |||
| 94 | 3-Biphenyl-3-yl-N-hydroxy-N-methyl-acrylamide | Drug Info | [153] | |||
| 95 | 3-Biphenyl-4-yl-N-hydroxy-N-methyl-acrylamide | Drug Info | [153] | |||
| 96 | 4,5-Dihydroxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 97 | 4,5-Dimethoxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 98 | 4-(1H-indol-3-yl)-1-morpholinobutan-1-one | Drug Info | [156] | |||
| 99 | 4-Bromo-N-hydroxy-benzamide | Drug Info | [153] | |||
| 100 | 4-Butoxy-N-hydroxy-N-methyl-benzamide | Drug Info | [153] | |||
| 101 | 4-Hydroxy-5-methoxy-10H-anthracen-9-one | Drug Info | [92] | |||
| 102 | 4-Pentadeca-1,3,6-trienylsulfanyl-butyric acid | Drug Info | [143] | |||
| 103 | 5,8-Dihydroxy-1,4-naphthoquinone | Drug Info | [92] | |||
| 104 | 5-Chloro-N-(4-ethylphenyl)benzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 105 | 5-Chloro-N-phenylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 106 | 5-Methoxy-N-phenylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 107 | 5-Methyl-2-p-tolyl-thiazol-4-ol | Drug Info | [155] | |||
| 108 | 5-Methyl-N-phenylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 109 | 5S-HETE | Drug Info | [84], [152] | |||
| 110 | 7-tert-butyl-2, 3-dihydro-3, 3-dimethyl substituted dihydrofuran 30 (DHDMBF30) | Drug Info | [158] | |||
| 111 | ACACETIN | Drug Info | [159] | |||
| 112 | Acanthus ilicifolius Linn | Drug Info | [137] | |||
| 113 | Acetic acid 2-phenyl-5-propyl-thiazol-4-yl ester | Drug Info | [155] | |||
| 114 | Acetic acid 5-butyl-2-phenyl-thiazol-4-yl ester | Drug Info | [155] | |||
| 115 | Anthracene-2-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 116 | ANTHRONE | Drug Info | [92] | |||
| 117 | Biphenyl-3-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 118 | Biphenyl-4-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 119 | BUDDLEDIN A | Drug Info | [159] | |||
| 120 | BW A360C | Drug Info | [136] | |||
| 121 | BW B218C | Drug Info | [136] | |||
| 122 | Chebulagic acid | Drug Info | [163] | |||
| 123 | CYLINDOL A | Drug Info | [164] | |||
| 124 | Heme | Drug Info | [166] | |||
| 125 | Hexanoic acid 2,5-diphenyl-thiazol-4-yl ester | Drug Info | [155] | |||
| 126 | Hyperforin | Drug Info | [167] | |||
| 127 | L-652,343 | Drug Info | [169] | |||
| 128 | N-(2-Ethylphenyl)-5-methylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 129 | N-(3-Bromophenyl)-5-methoxybenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 130 | N-(4-Ethylphenyl)-5-methylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 131 | N-(4-Ethylphenyl)benzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 132 | N-Hydroxy-2-methyl-3-naphthalen-2-yl-acrylamide | Drug Info | [153] | |||
| 133 | N-Hydroxy-2-naphthalen-2-yl-acetamide | Drug Info | [153] | |||
| 134 | N-Hydroxy-3-naphthalen-2-yl-acrylamide | Drug Info | [153] | |||
| 135 | N-Hydroxy-3-naphthalen-2-yl-N-p-tolyl-acrylamide | Drug Info | [153] | |||
| 136 | N-Hydroxy-3-naphthalen-2-yl-N-phenyl-acrylamide | Drug Info | [153] | |||
| 137 | N-Hydroxy-3-naphthalen-2-yl-propionamide | Drug Info | [153] | |||
| 138 | N-Hydroxy-3-phenyl-acrylamide | Drug Info | [153] | |||
| 139 | N-hydroxy-4-(naphthalen-1-yl)benzamide | Drug Info | [153] | |||
| 140 | N-Hydroxy-4-iodo-benzamide | Drug Info | [153] | |||
| 141 | N-Hydroxy-4-isobutyl-benzamide | Drug Info | [153] | |||
| 142 | N-Hydroxy-4-naphthalen-2-yl-benzamide | Drug Info | [153] | |||
| 143 | N-Hydroxy-N-methyl-2,3,3-triphenyl-acrylamide | Drug Info | [153] | |||
| 144 | N-Hydroxy-N-methyl-2-naphthalen-2-yl-propionamide | Drug Info | [153] | |||
| 145 | N-Hydroxy-N-methyl-3-naphthalen-1-yl-acrylamide | Drug Info | [153] | |||
| 146 | N-Hydroxy-N-methyl-3-naphthalen-2-yl-acrylamide | Drug Info | [103] | |||
| 147 | N-Hydroxy-N-methyl-3-naphthalen-2-yl-propionamide | Drug Info | [153] | |||
| 148 | N-Hydroxy-N-methyl-3-phenanthren-2-yl-acrylamide | Drug Info | [153] | |||
| 149 | N-Hydroxy-N-methyl-3-phenanthren-3-yl-acrylamide | Drug Info | [153] | |||
| 150 | N-Hydroxy-N-methyl-3-phenanthren-9-yl-acrylamide | Drug Info | [153] | |||
| 151 | N-Hydroxy-N-methyl-benzamide | Drug Info | [153] | |||
| 152 | N-hydroxy-N-[1-(4-isobutylphenyl)ethyl]urea | Drug Info | [170] | |||
| 153 | N-Phenylbenzo[d]oxazol-2-amine | Drug Info | [157] | |||
| 154 | Naphthalene-2-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 155 | Phenanthrene-2-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 156 | Phenanthrene-3-carboxylic acid hydroxyamide | Drug Info | [153] | |||
| 157 | PHENIDONE | Drug Info | [164] | |||
| 158 | PYROGALLOL | Drug Info | [92] | |||
| 159 | SK&F 107649 | Drug Info | [173] | |||
| 160 | TZI-41127 | Drug Info | [174] | |||
| Modulator | [+] 28 Modulator drugs | + | ||||
| 1 | Flobufen | Drug Info | [9] | |||
| 2 | FPL-62064 | Drug Info | [10] | |||
| 3 | Tenidap | Drug Info | [12] | |||
| 4 | Darbufelone | Drug Info | [13] | |||
| 5 | CMI-392 | Drug Info | [17] | |||
| 6 | E-6700 | Drug Info | [18] | |||
| 7 | MK-866 | Drug Info | [19] | |||
| 8 | Tipelukast | Drug Info | [101] | |||
| 9 | UCB-35440 | Drug Info | [102] | |||
| 10 | BF-389 | Drug Info | [30] | |||
| 11 | MK-886 | Drug Info | [113] | |||
| 12 | CI-986 | Drug Info | [63] | |||
| 13 | CMI-206 | Drug Info | [140] | |||
| 14 | ER-34122 | Drug Info | [66], [67] | |||
| 15 | KC-11404 | Drug Info | [68] | |||
| 16 | PD-146176 | Drug Info | [144] | |||
| 17 | RWJ-63556 | Drug Info | [73] | |||
| 18 | Sch-40120 | Drug Info | [146] | |||
| 19 | SKF-104351 | Drug Info | [147] | |||
| 20 | WY-28342 | Drug Info | [148] | |||
| 21 | ZD-7717 | Drug Info | [149] | |||
| 22 | BW-858C | Drug Info | [160] | |||
| 23 | BW-A137C | Drug Info | [161] | |||
| 24 | CGS-23885 | Drug Info | [162] | |||
| 25 | FR-122788 | Drug Info | [165] | |||
| 26 | ICI-211965 | Drug Info | [168] | |||
| 27 | SB-202235 | Drug Info | [171] | |||
| 28 | SC-41661A | Drug Info | [172] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | KC-11425 | Drug Info | [141] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ligand Name: Arachidonic acid | Ligand Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure Description | S663D Stable-5-LOX in complex with Arachidonic Acid | PDB:3V99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | X-ray diffraction | Resolution | 2.25 Å | Mutation | Yes | [175] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB Sequence |
SYTVTVATGS
14 QEHAGTDDYI24 YLSLVGSAGC34 SEKHLLDKGS44 FERGAVDSYD54 VTVDEELGEI 64 QLVRIEKRKY74 GSNDDWYLKY84 ITLKTPHGDY94 IEFPCYRWIT104 GDVEVVLRDG 114 RAKLARDDQI124 HILKQHRRKE134 LETRQKQYRW144 MEWNPGFPLS154 IDAKCHKDLP 164 RDIQFDSFVL179 NYSKAMENLF189 QSSWNDFADF207 EKIFVKISNT217 ISERVMNHWQ 227 EDLMFGYQFL237 NGANPVLIRR247 CTELPEKLPV257 TTEMVECSLE267 RQLSLEQEVQ 277 QGNIFIVDFE287 LLDGIDCTLQ303 FLAAPICLLY313 KNLANKIVPI323 AIQLNQIPGD 333 ENPIFLPSDA343 KYDWLLAKIW353 VRSSDFHVHQ363 TITHLLRTHL373 VSEVFGIAMY 383 RQLPAVHPIF393 KLLVAHVRFT403 IAINTKAREQ413 GGHVQMVQRA439 MKDLTYASLC 449 FPEAIKARGM459 ESKEDIPYYF469 YRDDGLLVWE479 AIRTFTAEVV489 DIYYEGDQVV 499 EEDPELQDFV509 NDVYVYGMRG519 RKSSGFPKSV529 KSREQLSEYL539 TVVIFTASAQ 549 HAAVNFGQYD559 WASWIPNAPP569 TMRAPPPTAK579 GVVTIEQIVD589 TLPDRGRSCW 599 HLGAVWALSQ609 FELFLGMYPE622 EHFIEKPVKE632 AMARFRKNLE642 AIVSVIAERN 652 ENLQLPYYYL662 DPDRIPNSVA672
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
☰Loading data... Dynamically generated for selected residues. Nodes can be dragged or clicked. Label: Selection: Name:
Note: VAST+ finds other macromolecular structures that have a similar biological unit. To do this, VAST+ takes into consideration the complete set of 3D domains that VAST identified within a query structure, throughout all of its component protein molecules, and finds other macromolecular structures that have a similar set of proteins/3D domains. PDB ID: Note: VAST identifies 3D domains (substructures) within each protein structure in the Molecular Modeling Database (MMDB), and then finds other protein structures that have one or more similar 3D domains, using purely geometric criteria. You have two ways to do a VAST search. Option 1, search with your selection (all residues are selected by default) in the loaded structures: Option 2, search with PDB ID and chain name: PDB ID: Chain Name: Option 3, search with a PDB file: 1. your selection (all residues are selected by default) in the loaded structures to Foldseek web server. 2 (Optional). Once you see the structure neighbors, you can view the alignment in iCn3D by inputing a list of PDB chain IDs or AlphaFold UniProt IDs below. The PDB chain IDs are the same as the record names such as "1HHO_A". The UniProt ID is the text between "AF-" and "-F1". For example, the UniProt ID for the record name "AF-P69905-F1-model_v4" is "P69905". Chain ID List: BCIF/MMTF ID: PDB ID: Note: AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100: Very high (pLDDT > 90) Confident (90 > pLDDT > 70) Low (70 > pLDDT > 50) Very low (pLDDT < 50) AlphaFold Uniprot ID: PAE Map: NCBI Protein Accession: Note: Several PDB files could be concatenated into a single PDB file. Use the line "ENDMDL" to separate PDB files. PDB File: Multiple PDB Files: The custom JSON file on residue colors has the following format for proteins("ALA" and "ARG") and nucleotides("G" and "A"): {"ALA":"#C8C8C8", "ARG":"#145AFF", ..., "G":"#008000", "A":"#6080FF", ...} Residue Color File: The custom file for the structure has two columns separated by space or tab: residue number, and score in the range of 0-100. If you click "Apply Custom Color" button, the scores 0, 50 and 100 correspond to the three colors specified below. If you click "Apply Custom Tube", the selected residues will be displayed in a style similar to "B-factor Tube". Custom File: 1. Score to Color: 0: 50: 100: or 2. You can define your own reference numbers in a custom file using Excel, and then export it as a CSV file. An example file is shown below with cells separated by commas. refnum,11,12,,21,22,,10C,11C,20CThe first row defines the reference residue numbers, which could be any strings. The 1st cell could be anything. The rest cells are reference residue numbers (e.g., 11, 21, 10C, etc.) or empty cells. Each chain has a separate row. The first cell of the second row is the chain ID "1TUP_A". The rest cells are the corresponding real residue numbers for reference residue numbers in the first row. For example, the reference numbers for residues 100, 101, and 132 in the chain 1TUP_A are 11, 12, and 22, respectively. The fourth row shows another set of reference numners for the chain "1TUP_C". It could be a chain from a different structure. To select all residues corresponding to the reference numbers, you can simplay replace ":" with "%" in the Specification. For example, "%12" selects the residue 101 in 1TUP_A and the residue 111 in 1TUP_B. ".A%12" has the chain "A" filter and selects the residue 101 in 1TUP_A. Custom File: Enter the PDB IDs or MMDB IDs of the structures: ID1: ID2: VAST+ based on VAST: VAST+ based on TM-align: All chains will be aligned to the first chain in the comma-separated chain IDs. Each chain ID has the form of PDBID_chain (e.g., 1HHO_A, case sensitive) or UniprotID (e.g., P69905 for AlphaFold structures). Chain IDs: (Note: To align chains in custom PDB files, you could load them in "File > Open File > PDB Files (appendable)" and click "Analysis > Defined Sets". Finally select multiple chains in Defined Sets and click "File > Realign Selection".) All chains will be aligned to the first chain in the comma-separated chain IDs. Each chain ID has the form of PDBID_chain (e.g., 1HHO_A, case sensitive) or UniprotID (e.g., P69905 for AlphaFold structures). Chain IDs: The sequence alignment (followed by structure alignment) is based on residue numbers in the First/Master chain: (Note: To align chains in custom PDB files, you could load them in "File > Open File > PDB Files (appendable)" and click "Analysis > Defined Sets". Finally select multiple chains in Defined Sets and click "File > Realign Selection".) All chains will be aligned to the first chain in the comma-separated chain IDs. Each chain ID has the form of PDBID_chain (e.g., 1HHO_A, case sensitive) or UniprotID (e.g., P69905 for AlphaFold structures). Chain IDs: Each alignment is defined as " | "-separated residue lists in one line. "10-50" means a range of residues from 10 to 50. Option 1: Option 2: All chains will be aligned to the first chain in the comma-separated chain IDs. Each chain ID has the form of PDBID_chain (e.g., 1HHO_A, case sensitive) or UniprotID (e.g., P69905 for AlphaFold structures). Chain IDs: Each alignment is defined as " | "-separated residue lists in one line. "10-50" means a range of residues from 10 to 50. Please specify the mutations with a comma separated mutation list. Each mutation can be specified as "[uppercase PDB ID or AlphaFold UniProt ID]_[Chain Name]_[Residue Number]_[One Letter Mutant Residue]". E.g., the mutation of N501Y in the E chain of PDB 6M0J can be specified as "6M0J_E_501_Y". For AlphaFold structures, the "Chain ID" is "A". If you load a custom structure without PDB or UniProt ID, you can open "Seq. & Annotations" window and find the chain ID such as "stru_A". The part before the underscore is the structure ID, which can be used to specify the mutation such as "stru_A_...". Remember to choose "Show Mutation in: Current Page". Mutations: ID Type: PDB IDAlphaFold UniProt ID Show Mutation in: Current PageNew Page Mol2 File: SDF File: XYZ File: AlphaFold PAE File: File type: URL in the same host: Multiple mmCIF Files: mmCIF ID: MMDB or PDB ID: Note: The "biological unit" is the biochemically active form of a biomolecule, List of PDB, MMDB, or AlphaFold UniProt structures: or Note: The "biological unit" is the biochemically active form of a biomolecule, Enter a protein sequence ID (or FASTA sequence) and the aligned protein accession, which can be found using the BLAST search with the protein sequence ID or FASTA sequence as input. If the protein accession is not a PDB chain, the corresponding AlphaFold UniProt structure is used. Protein Sequence ID(NCBI protein accession of a sequence): or FASTA sequence: Aligned Protein Accession (or a chain of a PDB): The sequence to structure prediction is done via ESM Metagenomic Atlas. The sequence should be less than 400 characters. For any sequence longer than 400, please see the discussion here. FASTA sequence: Your note will be saved in the HTML file when you click "File > Save File > iCn3D PNG Image". Protein/Gene name: PubChem CID/Name/InchI: Chemical SMILES: Multiple iCn3D PNG images: State file: Since January 6, 2021, you can show the original view with the archived version of iCn3D by pasting your URL below and click "Show Originial View". Note the version in the parameter "v" was used to replace "full.html" with "full_[v].html" in the URL. Share Link URL: Selection file: Collection File: Structures: Note: Always load a PDB file before loading map files. If you don't specify the threshold below, a default one will be chosen. 2fofc contour at default threshold or at: σ fofc contour at default threshold or at: σ Note: Always load a PDB file before loading map files. If you don't specify the threshold below, a default one will be chosen. 2fofc contour at default threshold or at: σ URL in the same host: fofc contour at default threshold or at: σ URL in the same host: Click in the input box to use the color picker: Custom Color: Grid Size: Salt Concentration: M Potential contour at: kT/e(25.6mV at 298K) Note: Only the selected residues are used for DelPhi potential calculation by solving linear Poisson-Boltzmann equation. Grid Size: Salt Concentration: M Surface with max potential at: kT/e(25.6mV at 298K) Surface: Opacity: Wireframe: Note: Only the selected residues are used for DelPhi potential calculation by solving linear Poisson-Boltzmann equation. Potential contour at: kT/e(25.6mV at 298K) Note: Always load a PDB file before loading a PQR or DelPhi potential file. Potential contour at: kT/e(25.6mV at 298K) Grid Size: Salt Concentration: M PQR URL in the same host: Phi URL in the same host: Cube URL in the same host: Note: Always load a PDB file before loading a PQR or DelPhi potential file. Symmetry: Distance: Contact Type: 1. Choose interaction types and their thresholds:
4. Sort Interactions on: to show two lines of residue nodes to show map with atom details to show interactions with strength parameters in 0-200:
(Note: you can also adjust thresholds at #1 to add/remove interactions.) 5. and select new sets 1. Select sets below or use your current selection: 2. 1. Select sets below or use your current selection. 2. 1. Select sets below or use your current selection: 2. Overall maximum RMSD: Å 3. 1. Select sets below: 2. 1. Select sets below: 2. 1. Select sets below: 2. 1. Select sets below: 2. Hold Ctrl key to select multiple nodes/lines. Green: H-Bonds; Cyan: Salt Bridge/Ionic; Grey: Contacts Magenta: Halogen Bonds; Red: π-Cation; Blue: π-Stacking Scale: Hold Ctrl key to select multiple nodes. Scale: Note: Nodes/Residues can be dragged. Both nodes and dashed lines/interactions can be clicked to select residues. Color legend for interactions (dashed lines): Green: H-Bonds; Cyan: Salt Bridge/Ionic; Grey: Contacts Magenta: Halogen Bonds; Red: π-Cation; Blue: π-Stacking Scale: Hold Ctrl key to select multiple nodes. Scale: Hold Ctrl key to select multiple nodes. Scale:
Contour at: σ Contour at: σ Contour at: % of maximum EM values 1. Select the first set: 2. Sphere with a radius: Å 3. Select the second set to apply the sphere: 4. the sphere around the first set of atoms interacting/contacting residue pairs in a file Note: The membranes are parallel to the X-Y plane. The center of the membranes is at Z = 0. 1. Extracellular membrane Z-axis position: Å 2. intracellular membrane Z-axis position: Å 3. the adjusted membranes Note: The membranes are parallel to the X-Y plane. The center of the membranes is at Z = 0. 1. Z-axis position of the first X-Y plane: Å 2. Z-axis position of the second X-Y plane: Å 3. the region between the planes to Defined Sets 1. Text: 2. Size: 3. Color: 4. Pick TWO atoms while holding "Alt" key 5. 1. Text: 2. Size: 3. Color: 4. Color for all labels: 1. Pick TWO atoms while holding "Alt" key 2. Line Color: 3. 1. Pick TWO atoms while holding "Alt" key 2. Color: 3. 1. Select two sets
3. 1. Select two sets
2. Line style: 3. Line radius: 4. Color: 5. Opacity: 6. 1. Select a set: 2. Shape: 3. Radius: 4. Color: 5. Opacity: 6. 1. Select sets for pairwise distances
Note: Each set is represented by a vector, which is the X-axis of the principle axes. The angles between the vectors are then calculated. 1. Select sets for pairwise angles
1. Pick TWO atoms while holding "Alt" key 2. Line Radius: (for stabilizers, hydrogen bonds, distance lines, default 0.1) Coil Radius: (for coils, default 0.3) Stick Radius: (for sticks, default 0.4) Cross-Linkage Radius: (for cross-linkages, default 0.4) Trace Radius: (for C alpha trace, O3' trace, default 0.4) Ribbon Thickness: (for helix and sheet ribbons, nucleotide ribbons, default 0.2) Protein Ribbon Width: (for helix and sheet ribbons, default 1.3) Nucleotide Ribbon Width: (for nucleotide ribbons, default 0.8) Ball Scale: (for styles 'Ball and Stick' and 'Dot', default 0.3) Note: The following parameters will be saved in cache. You just need to set them once. 1. Shininess: (for the shininess of the 3D objects, default 40) 2. Three directional lights: Key Light: (for the light strength of the key light, default 0.8) Fill Light: (for the light strength of the fill light, default 0.4) Back Light: (for the light strength of the back light, default 0.2) 3. Thickness: Line Radius: (for stabilizers, hydrogen bonds, distance lines, default 0.1) Coil Radius: (for coils, default 0.3) Stick Radius: (for sticks, default 0.4) Cross-Linkage Radius: (for cross-linkages, default 0.4) Trace Radius: (for C alpha trace, O3' trace, default 0.4) Ribbon Thickness: (for helix and sheet ribbons, nucleotide ribbons, default 0.2) Protein Ribbon Width: (for helix and sheet ribbons, default 1.3) Nucleotide Ribbon Width: (for nucleotide ribbons, default 0.8) Ball Scale: (for styles 'Ball and Stick' and 'Dot', default 0.3) 4. Show Glycan Cartoon: (0: hide, 1: show, default 0) 5. Show Membrane: (0: hide, 1: show, default 1) 6. Enlarge Command Window: (0: Regular, 1: Large, default 0) Name: 1. URLs Used in Browsers Please copy one of the URLs below. They show the same result. (To add a title to share link, click "Windows > Your Note" and click "File > Share Link" again.) Original URL with commands: Lifelong Short URL:(To replace this URL, send a pull request to update share.html at iCn3D GitHub) Lifelong Short URL + Window Title:(To update the window title, click "Analysis > Your Note/Window Title".) 2. Commands Used in Jupyter Noteboook Please copy the following commands into a cell in Jupyter Notebook to show the same result. More details are at https://github.com/ncbi/icn3d/tree/master/jupyternotebook. Annotations:
Zoom: mouse wheel; Move: left button; Select Multiple Nodes: Ctrl Key and drag an Area Force on Nodes: Label Size: Internal Edges: Solvent Accessible Surface Area(SASA) calculated using the EDTSurf algorithm: (0-20% out is considered "in". 50-100% out is considered "out".) Toal: Å2 Color each residue based on the percentage of solvent accessilbe surface area. The color ranges from blue, to white, to red for a percentage of 0, 35(variable), and 100, respectively. Middle Percentage(White): % Select residue based on the percentage of solvent accessilbe surface area. The values are in the range of 0-100. Min Percentage: % Max Percentage: % Select residue based on B-factor/pLDDT. The values are in the range of 0-100. Min B-factor/pLDDT: % Max B-factor/pLDDT: % X: Y: Z: Vector 1, X: Y: Z: Vector 2, X: Y: Z: The angle is: degree. 0: 4: 8: 12: 1: 5: 9: 13: 2: 6: 10: 14: 3: 7: 11: 15: Choose an Ig template for selected residues: Choose an Ig template to align with selected residues: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
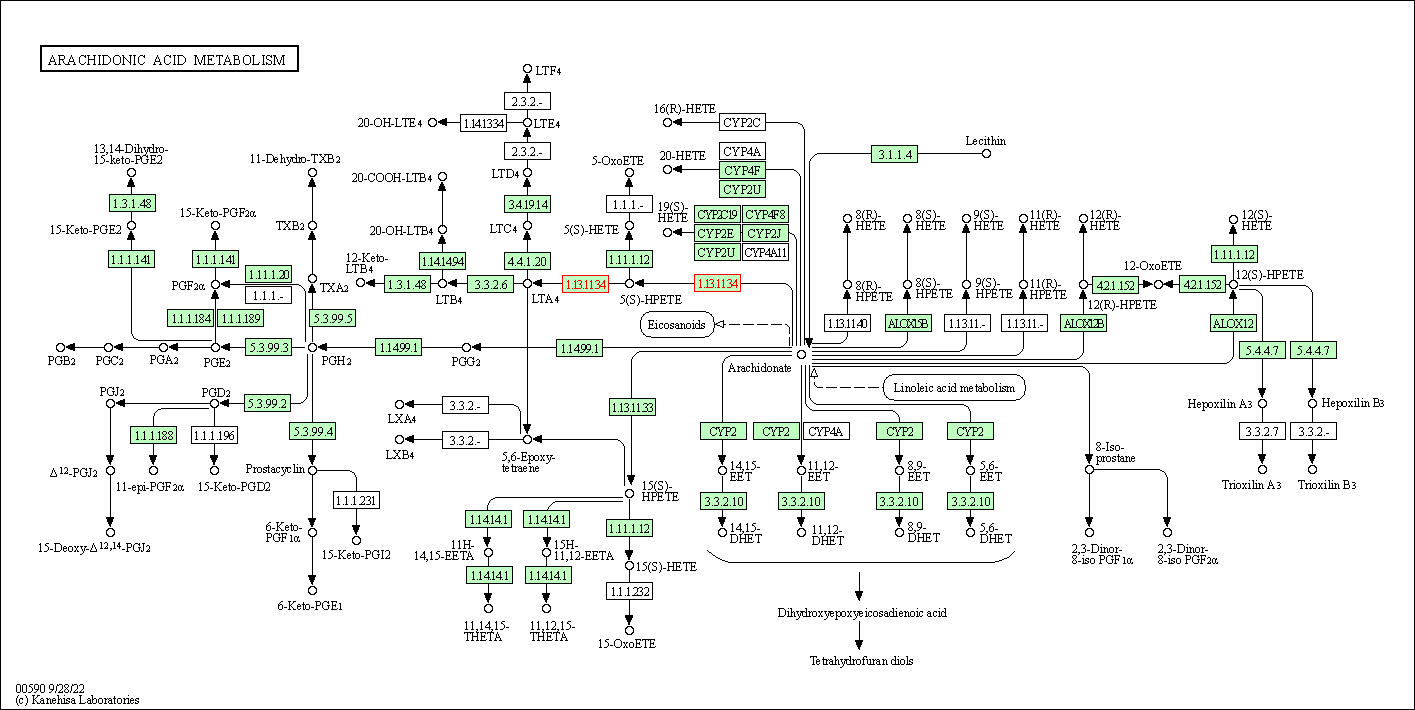
| Arachidonic acid metabolism | hsa00590 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
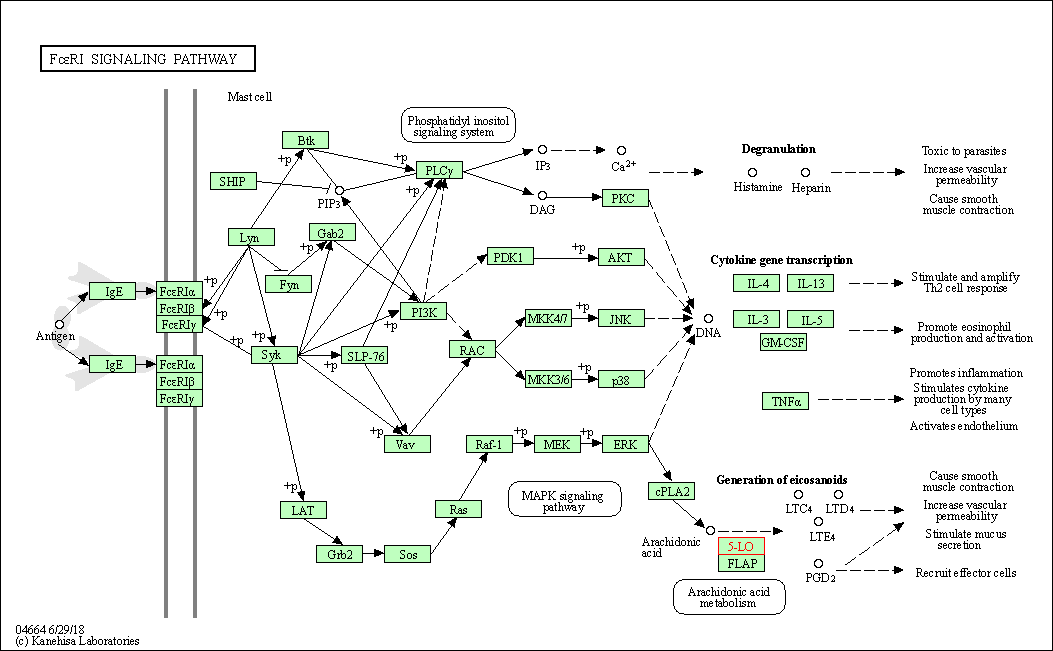
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
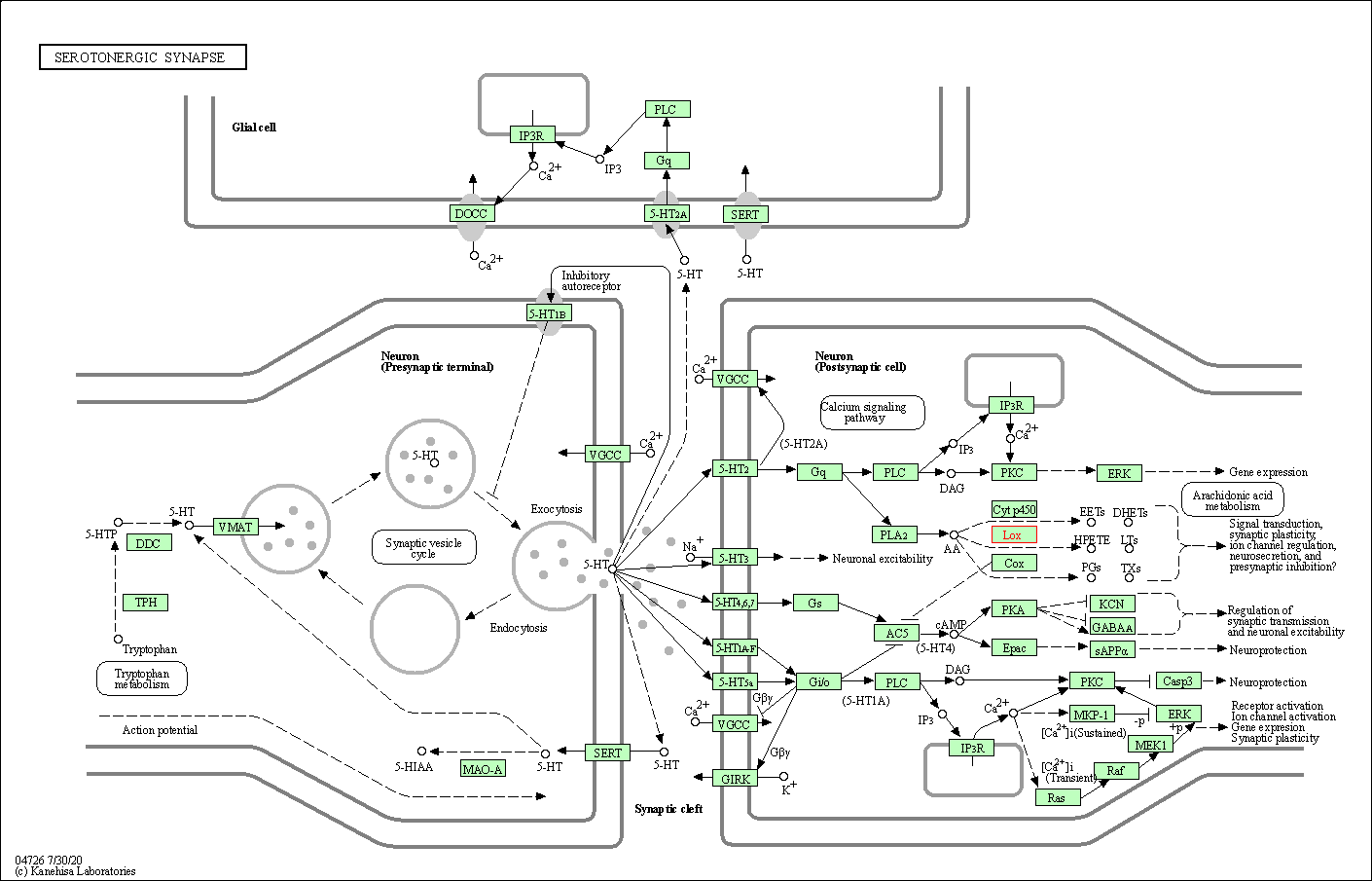
| Serotonergic synapse | hsa04726 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
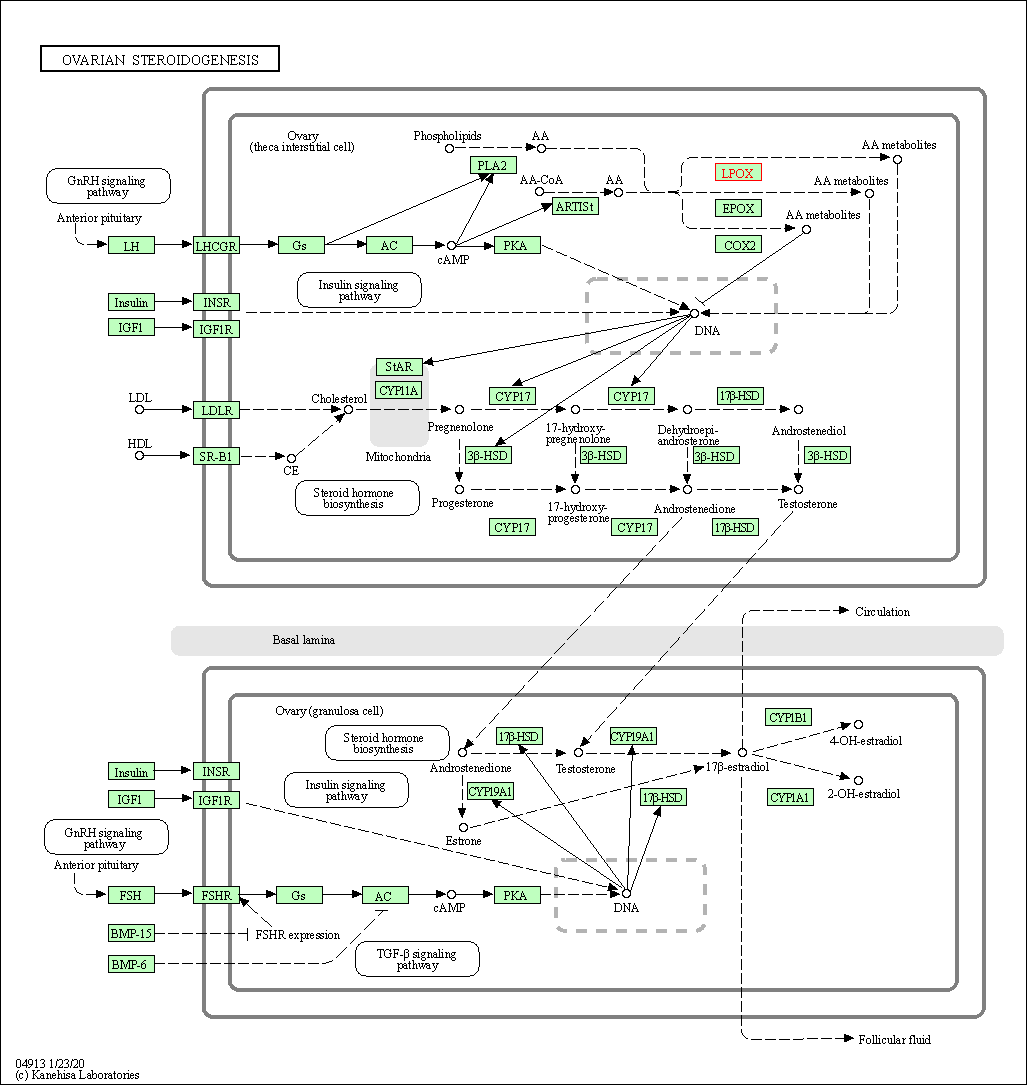
| Ovarian steroidogenesis | hsa04913 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 7 | Degree centrality | 7.52E-04 | Betweenness centrality | 8.13E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.93E-01 | Radiality | 1.33E+01 | Clustering coefficient | 4.76E-02 |
| Neighborhood connectivity | 4.71E+00 | Topological coefficient | 2.11E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 6 BioCyc Pathways | + | ||||
| 1 | Aspirin-triggered lipoxin biosynthesis | |||||
| 2 | Resolvin D biosynthesis | |||||
| 3 | Leukotriene biosynthesis | |||||
| 4 | Lipoxin biosynthesis | |||||
| 5 | Aspirin triggered resolvin D biosynthesis | |||||
| 6 | Aspirin triggered resolvin E biosynthesis | |||||
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Arachidonic acid metabolism | |||||
| 2 | Metabolic pathways | |||||
| 3 | Serotonergic synapse | |||||
| 4 | Ovarian steroidogenesis | |||||
| 5 | Toxoplasmosis | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Arachidonic Acid Metabolism | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Vitamin D Receptor Pathway | |||||
| 2 | Arachidonic acid metabolism | |||||
| 3 | Eicosanoid Synthesis | |||||
| 4 | Selenium Micronutrient Network | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Inhibition of leukotriene formation by diethylcarbamazine modifies the acid-base balance in the rabbits with blast injuries of the lungs. Vojnosanit Pregl. 1999 May-Jun;56(3):243-7. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005 Sep;4(9):727-40. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5297). | |||||
| REF 5 | Current and emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs. 2007 Nov;12(4):627-46. | |||||
| REF 6 | ClinicalTrials.gov (NCT02556814) Caffeic Acid Combining High-dose Dexamethasone in Management of ITP. U.S. National Institutes of Health. | |||||
| REF 7 | ABT-761 (Abbott). Curr Opin Investig Drugs. 2001 Jan;2(1):68-71. | |||||
| REF 8 | Clinical pipeline report, company report or official report of Roche. | |||||
| REF 9 | Pharmacological profile of the novel potent antirheumatic 4-(2',4'-difluorobiphenyl-4-yl)-2-methyl-4-oxobutanoic acid. Arzneimittelforschung. 1997 May;47(5):648-52. | |||||
| REF 10 | FPL 62064, a topically active 5-lipoxygenase/cyclooxygenase inhibitor. Agents Actions. 1990 Jun;30(3-4):432-42. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2395). | |||||
| REF 12 | The in vitro free radical scavenging activity of tenidap, a new dual cyclo-oxygenase and 5-1ipoxygenase inhibitor. Mediators Inflamm. 1992;1(2):141-3. | |||||
| REF 13 | Novel dual cyclooxygenase and lipoxygenase inhibitors targeting hyaluronan-CD44v6 pathway and inducing cytotoxicity in colon cancer cells. Bioorg Med Chem. 2013 May 1;21(9):2551-9. | |||||
| REF 14 | ClinicalTrials.gov (NCT03830684) A Randomized, Double-blind, Placebo-controlled, Multicenter and Phase IIa Clinical Trial for the Effectiveness and Safety of Baicalein Tablets in the Treatment of Improve Other Aspects of Healthy Adult With Influenza Fever. U.S. National Institutes of Health. | |||||
| REF 15 | BIM-23A760, a chimeric molecule directed towards somatostatin and dopamine receptors, vs universal somatostatin receptors ligands in GH-secreting pituitary adenomas partial responders to octreotide. J Endocrinol Invest. 2005;28(11 Suppl International):21-7. | |||||
| REF 16 | Emerging drugs for acromegaly. Expert Opin Emerg Drugs. 2008 Jun;13(2):273-93. | |||||
| REF 17 | Anti-inflammatory activities of LDP-392, a dual PAF receptor antagonist and 5-lipoxygenase inhibitor. Pharmacol Res. 2001 Sep;44(3):213-20. | |||||
| REF 18 | Structure-activity relationships of (E)-3-(1,4-benzoquinonyl)-2-[(3-pyridyl)-alkyl]-2-propenoic acid derivatives that inhibit both 5-lipoxygenase and thromboxane A2 synthetase. J Med Chem. 1996 Aug 2;39(16):3148-57. | |||||
| REF 19 | Inhibition of leukotriene synthesis with MK-886 prevents a rise in blood pressure and reduces noradrenaline-evoked contraction in L-NAME-treated rats | |||||
| REF 20 | ClinicalTrials.gov (NCT01147458) A Study Of The Safety And Efficacy Of PF-04191834 In Patients With Osteoarthritis Of The Knee. U.S. National Institutes of Health. | |||||
| REF 21 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6040). | |||||
| REF 22 | The effects of antiinflammatory and antiallergic drugs on cytokine release after stimulation of human whole blood by lipopolysaccharide and zymosan A. Inflamm Res. 1995 Jul;44(7):269-74. | |||||
| REF 23 | ClinicalTrials.gov (NCT03571620) Safety and Efficacy Study of Q301 in Mild to Moderate Adolescents and Adults Atopic Dermatitis Patients. U.S. National Institutes of Health. | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006112) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010788) | |||||
| REF 26 | Effects of tepoxalin, a dual inhibitor of cyclooxygenase/5-lipoxygenase, on events associated with NSAID-induced gastrointestinal inflammation. Prostaglandins Leukot Essent Fatty Acids. 1997 Jun;56(6):417-23. | |||||
| REF 27 | ClinicalTrials.gov (NCT02503657) Safety and Tolerability Study in Subjects With Idiopathic Pulmonary Fibrosis (IPF). | |||||
| REF 28 | Clinical pipeline report, company report or official report of UCB. | |||||
| REF 29 | WY-50295 tromethamine: a 5-lipoxygenase inhibitor without activity in human whole blood. Prostaglandins Leukot Essent Fatty Acids. 1999 Jan;60(1):31-41. | |||||
| REF 30 | Antiarthritic profile of BF-389--a novel anti-inflammatory agent with low ulcerogenic liability. Agents Actions. 1992 Sep;37(1-2):90-8. | |||||
| REF 31 | Analgetic activity of SK&F 105809, a dual inhibitor of arachidonic acid metabolism. Agents Actions Suppl. 1991;32:113-7. | |||||
| REF 32 | Anti-inflammatory agents: determination of ibuproxam and its metabolite humans. Correlation between bioavailability, tolerance and chemico-physical characteristics. Arzneimittelforschung. 1980;30(9):1607-9. | |||||
| REF 33 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5169). | |||||
| REF 34 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003690) | |||||
| REF 36 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005994) | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001952) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006733) | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005063) | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002311) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001712) | |||||
| REF 42 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2655). | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000073) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007577) | |||||
| REF 45 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2196). | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000284) | |||||
| REF 47 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003630) | |||||
| REF 48 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001362) | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002346) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009803) | |||||
| REF 51 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008118) | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003927) | |||||
| REF 53 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003598) | |||||
| REF 54 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004773) | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003607) | |||||
| REF 56 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000099) | |||||
| REF 57 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002162) | |||||
| REF 58 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005261) | |||||
| REF 59 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000230) | |||||
| REF 60 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002163) | |||||
| REF 61 | BW755C, a dual lipoxygenase/cyclooxygenase inhibitor, reduces mural platelet and neutrophil deposition and vasoconstriction after angioplasty injury in pigs. J Pharmacol Exp Ther. 1996 Apr;277(1):17-21. | |||||
| REF 62 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005833) | |||||
| REF 63 | Role of leukotrienes on coronary vasoconstriction in isolated hearts of arthritic rats: effect of in vivo treatment with CI-986, a dual inhibitor of cyclooxygenase and lipoxygenase. Pharmacology. 2000 Jan;60(1):41-6. | |||||
| REF 64 | Cmi206: A potent dual platelet activating factor antagonist and 5-lipoxygenase inhibitor. Bioorganic & Medicinal Chemistry Letters. 03/1995; 5(6):643-648. | |||||
| REF 65 | Epocarbazolins A and B, novel 5-lipoxygenase inhibitors. Taxonomy, fermentation, isolation, structures and biological activities. J Antibiot (Tokyo). 1993 Jan;46(1):25-33. | |||||
| REF 66 | Improvement of dissolution and oral absorption of ER-34122, a poorly water-soluble dual 5-lipoxygenase/cyclooxygenase inhibitor with anti-inflammatory activity by preparing solid dispersion. J Pharm Sci. 2002 Jan;91(1):258-66. | |||||
| REF 67 | ER-34122, a novel dual 5-lipoxygenase/cyclooxygenase inhibitor with potent anti-inflammatory activity in an arachidonic acid-induced ear inflammation model. Inflamm Res. 1998 Oct;47(10):375-83. | |||||
| REF 68 | Synthesis, structure-activity relationships, and pharmacological evaluation of pyrrolo[3,2,1-ij]quinoline derivatives: potent histamine and platelet activating factor antagonism and 5-lipoxygenase inhibitory properties. Potential therapeutic application in asthma. J Med Chem. 1995 Feb 17;38(4):669-85. | |||||
| REF 69 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005461) | |||||
| REF 70 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002348) | |||||
| REF 71 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010267) | |||||
| REF 72 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026867) | |||||
| REF 73 | Evaluation of the antiinflammatory activity of a dual cyclooxygenase-2 selective/5-lipoxygenase inhibitor, RWJ 63556, in a canine model of inflammation. J Pharmacol Exp Ther. 1997 Aug;282(2):1094-101. | |||||
| REF 74 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003693) | |||||
| REF 75 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000933) | |||||
| REF 76 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005582) | |||||
| REF 77 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007338) | |||||
| REF 78 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005134) | |||||
| REF 79 | 5-lipoxygenase inhibitor zileuton attenuates ischemic brain damage: involvement of matrix metalloproteinase 9. Neurol Res. 2009 Oct;31(8):848-52. | |||||
| REF 80 | 5-lipoxygenase pharmacogenetics in asthma: overlap with Cys-leukotriene receptor antagonist loci. Pharmacogenet Genomics. 2009 Mar;19(3):244-7. | |||||
| REF 81 | Oxygen-glucose deprivation activates 5-lipoxygenase mediated by oxidative stress through the p38 mitogen-activated protein kinase pathway in PC12 cells. J Neurosci Res. 2009 Mar;87(4):991-1001. | |||||
| REF 82 | Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008 Oct 15;14(20):6525-30. | |||||
| REF 83 | Leukotrienes in respiratory disease. Paediatr Respir Rev. 2001 Sep;2(3):238-44. | |||||
| REF 84 | Preview of potential therapeutic applications of leukotriene B4 inhibitors in dermatology. Skin Pharmacol Appl Skin Physiol. 2000 Sep-Oct;13(5):235-45. | |||||
| REF 85 | Phenidone protects the nigral dopaminergic neurons from LPS-induced neurotoxicity. Neurosci Lett. 2008 Nov 7;445(1):1-6. | |||||
| REF 86 | Protection of mouse brain from aluminum-induced damage by caffeic acid. CNS Neurosci Ther. 2008 Spring;14(1):10-6. | |||||
| REF 87 | Involvement of the 5-lipoxygenase pathway in the neurotoxicity of the prion peptide PrP106-126. J Neurosci Res. 2001 Sep 15;65(6):565-72. | |||||
| REF 88 | Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicine. 2000 Mar;7(1):21-4. | |||||
| REF 89 | Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging. 2010 May;3(3):298-307. | |||||
| REF 90 | The novel 5-lipoxygenase inhibitor ABT-761 attenuates cerebral vasospasm in a rabbit model of subarachnoid hemorrhage. Neurosurgery. 2001 Nov;49(5):1205-12; discussion 1212-3. | |||||
| REF 91 | N-hydroxyurea and hydroxamic acid inhibitors of cyclooxygenase and 5-lipoxygenase. Bioorg Med Chem Lett. 1999 Apr 5;9(7):979-84. | |||||
| REF 92 | Simple analogues of anthralin: unusual specificity of structure and antiproliferative activity. J Med Chem. 1997 Nov 7;40(23):3773-80. | |||||
| REF 93 | Indazolinones, a new series of redox-active 5-lipoxygenase inhibitors with built-in selectivity and oral activity. J Med Chem. 1991 Mar;34(3):1028-36. | |||||
| REF 94 | Pharmacologic and clinical effects of lonapalene (RS 43179), a 5-lipoxygenase inhibitor, in psoriasis. J Invest Dermatol. 1990 Jul;95(1):50-4. | |||||
| REF 95 | Pharmacology of PF-4191834, a novel, selective non-redox 5-lipoxygenase inhibitor effective in inflammation and pain. J Pharmacol Exp Ther. 2010 Jul;334(1):294-301. | |||||
| REF 96 | Therapeutic intervention in a rat model of ARDS: I. Dual inhibition of arachidonic acid metabolism. Circ Shock. 1990 Nov;32(3):231-42. | |||||
| REF 97 | Clinical pipeline report, company report or official report of Qurient. | |||||
| REF 98 | Therapy of seborrheic eczema with an antifungal agent with an antiphlogistic effect. Mycoses. 1991;34 Suppl 1:91-3. | |||||
| REF 99 | TA-270 [4-hydroxy-1-methyl-3-octyloxy-7-sinapinoylamino-2(1H)-quinolinone], an anti-asthmatic agent, inhibits leukotriene production induced by IgE... J Pharmacol Exp Ther. 2003 Nov;307(2):583-8. | |||||
| REF 100 | Preclinical toxicity evaluation of tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, in Sprague-Dawley rats and beagle dogs. Fundam Appl Toxicol. 1996 Sep;33(1):38-48. | |||||
| REF 101 | Company report (MediciNova) | |||||
| REF 102 | The effect of a novel, dual function histamine H1 receptor antagonist/5-lipoxygenase enzyme inhibitor on in vivo dermal inflammation and extravasat... Eur J Pharmacol. 2005 Jan 4;506(3):265-71. | |||||
| REF 103 | In vivo characterization of hydroxamic acid inhibitors of 5-lipoxygenase. J Med Chem. 1987 Nov;30(11):2121-6. | |||||
| REF 104 | A phase II study of the 5-lipoxygenase inhibitor, CV6504, in advanced pancreatic cancer: correlation of clinical data with pharmacokinetic and pharmacodynamic endpoints. Ann Oncol. 2000 Sep;11(9):1165-70. | |||||
| REF 105 | Involvement of thromboxane A2, leukotrienes and free radicals in puromycin nephrosis in rats. Kidney Int. 1991 May;39(5):920-9. | |||||
| REF 106 | The lipoxygenase inhibitor 2-phenylmethyl-1-naphthol (DuP 654) is a 12(S)-hydroxyeicosatetraenoic acid receptor antagonist in the human epidermal cell line SCL-II. Skin Pharmacol. 1993;6(2):148-51. | |||||
| REF 107 | In vitro effects of E3040, a dual inhibitor of 5-lipoxygenase and thromboxane A(2) synthetase, on eicosanoid production. Eur J Pharmacol. 2001 Jun 22;422(1-3):209-16. | |||||
| REF 108 | Effects of the new 5-lipoxygenase inhibitor E6080 on leukotriene release in vitro. Int Arch Allergy Immunol. 1992;97(4):267-73. | |||||
| REF 109 | Selective blockade of leukotriene production by a single dose of the FPL 64170XX 0.5% enema in active ulcerative colitis. Pharmacol Toxicol. 1995 Dec;77(6):371-6. | |||||
| REF 110 | Effects of TMK688, a novel anti-allergic drug, on allergic nasal obstruction and exudative responses in sensitized guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 1997 Dec;356(6):815-9. | |||||
| REF 111 | Antileukotriene therapy for asthma. Am J Health Syst Pharm. 1996 Dec 1;53(23):2821-30; quiz 2877-8. | |||||
| REF 112 | Inhibition of antigen-induced contraction of guinea-pig airways by a leukotriene synthesis inhibitor, BAY x1005. Eur J Pharmacol. 1994 Jun 2;258(1-2):95-102. | |||||
| REF 113 | Inhibitory effects of MK-886 on arachidonic acid metabolism in human phagocytes. Br J Pharmacol. 1990 May;100(1):15-20. | |||||
| REF 114 | Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers (Basel) 2014 September; 6(3): 1500-1521. | |||||
| REF 115 | Fuscoside: an anti-inflammatory marine natural product which selectively inhibits 5-lipoxygenase. Part II: Biochemical studies in the human neutrophil. J Pharmacol Exp Ther. 1992 Aug;262(2):874-82. | |||||
| REF 116 | Topical R-85355, a potent and selective 5-lipoxygenase inhibitor, fails to improve psoriasis. Skin Pharmacol. 1996;9(5):307-11. | |||||
| REF 117 | The immunosuppressive properties of enisoprost and a 5-lipoxygenase inhibitor (SC-45662). Transplantation. 1991 Dec;52(6):1053-7. | |||||
| REF 118 | New cyclooxygenase-2/5-lipoxygenase inhibitors. 2. 7-tert-butyl-2,3-dihydro-3,3-dimethylbenzofuran derivatives as gastrointestinal safe antiinflamm... J Med Chem. 1998 Mar 26;41(7):1124-37. | |||||
| REF 119 | Palladium catalyzed aryl(alkyl)thiolation of unactivated arenes. Org Lett. 2014 Feb 7;16(3):848-51. | |||||
| REF 120 | Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) selectivity of COX inhibitors. Prostaglandins Leukot Essent Fatty Acids. 2008 Feb;78(2):99-108. | |||||
| REF 121 | Activity and potential role of licofelone in the management of osteoarthritis. Clin Interv Aging. 2007;2(1):73-9. | |||||
| REF 122 | Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mitochondrial pathway independently from its abil... Carcinogenesis. 2008 Feb;29(2):371-80. | |||||
| REF 123 | Licofelone (ML-3000), a dual inhibitor of 5-lipoxygenase and cyclooxygenase, reduces the level of cartilage chondrocyte death in vivo in experimental dog osteoarthritis: inhibition of pro-apoptotic factors. J Rheumatol. 2002 Jul;29(7):1446-53. | |||||
| REF 124 | (+/-)-trans-2-[3-methoxy-4-(4-chlorophenylthioethoxy)-5-(N-methyl-N- hydroxyureidyl)methylphenyl]-5-(3,4, 5-trimethoxyphenyl)tetrahydrofuran (CMI-3... J Med Chem. 1998 May 21;41(11):1970-9. | |||||
| REF 125 | The role of platelet-activating factor (PAF) and the efficacy of ABT-491, a highly potent and selective PAF antagonist, in experimental allergic rhinitis. J Pharmacol Exp Ther. 1998 Jan;284(1):83-8. | |||||
| REF 126 | Stereoselective metabolism of the 5-lipoxygenase inhibitor A-78773. Ann N Y Acad Sci. 1994 Nov 15;744:262-73. | |||||
| REF 127 | CA patent application no. 445634, Acne treatment comprising lipoxygenase inhibitors. | |||||
| REF 128 | Platelets stimulate airway smooth muscle cell proliferation through mechanisms involving 5-lipoxygenase and reactive oxygen species. Platelets. 2008 Nov;19(7):528-36. | |||||
| REF 129 | Leukotriene B4/leukotriene B4 receptor pathway is involved in hepatic microcirculatory dysfunction elicited by endotoxin. Shock. 2008 Jul;30(1):87-91. | |||||
| REF 130 | Effect of 5-lipoxygenase inhibitor on experimental delayed cerebral vasospasm. Stroke. 1987 Mar-Apr;18(2):512-8. | |||||
| REF 131 | Characterization and modulation of antigen-induced effects in isolated rat heart. J Cardiovasc Pharmacol. 1991 Oct;18(4):556-65. | |||||
| REF 132 | A prodrug of a 2,6-disubstituted 4-(2-arylethenyl)phenol is a selective and orally active 5-lipoxygenase inhibitor. J Pharmacol Exp Ther. 1993 May;265(2):483-9. | |||||
| REF 133 | 5-Hydroxyanthranilic acid derivatives as potent 5-lipoxygenase inhibitors. J Antibiot (Tokyo). 1993 May;46(5):705-11. | |||||
| REF 134 | Reactive oxygen species and lipoxygenases regulate the oncogenicity of NPM-ALK-positive anaplastic large cell lymphomas. Oncogene. 2009 Jul 23;28(29):2690-6. | |||||
| REF 135 | Tumour necrosis factor production in a rat airpouch model of inflammation: role of eicosanoids. Agents Actions. 1991 Mar;32(3-4):289-94. | |||||
| REF 136 | Hydroxamic acids and hydroxyureas as novel, selective 5-lipoxygenase inhibitors for possible use in asthma. Agents Actions Suppl. 1991;34:189-99. | |||||
| REF 137 | Anti-inflammatory activity of Acanthus ilicifolius. J Ethnopharmacol. 2008 Oct 30;120(1):7-12. | |||||
| REF 138 | Characterization of CGS 8515 as a selective 5-lipoxygenase inhibitor using in vitro and in vivo models. Biochim Biophys Acta. 1988 Apr 15;959(3):332-42. | |||||
| REF 139 | CGS 26529: the biological profile of a novel, orally active 5-lipoxygenase inhibitor with an extended duration of action. Inflamm Res. 1995 Aug;44 Suppl 2:S147-8. | |||||
| REF 140 | DOI: 10.1016/0960-894X(95)00088-B | |||||
| REF 141 | WO patent application no. 2002,01223,5, 1,4-dihydropyridines as bradykinin antagonists. | |||||
| REF 142 | Anti-inflammatory effects of LY221068 and LY269415. Agents Actions. 1991 Sep;34(1-2):100-2. | |||||
| REF 143 | Design, synthesis, and 5-lipoxygenase-inhibiting properties of 1-thio-substituted butadienes. J Med Chem. 1990 Apr;33(4):1163-70. | |||||
| REF 144 | A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit.Atherosclerosis.1998 Feb;136(2):203-16. | |||||
| REF 145 | Mechanism-based inhibition of human liver microsomal cytochrome P450 1A2 by zileuton, a 5-lipoxygenase inhibitor. Drug Metab Dispos. 2003 Nov;31(11):1352-60. | |||||
| REF 146 | Actions of a 5-lipoxygenase inhibitor, Sch 40120, on acute inflammatory responses. J Pharmacol Exp Ther. 1992 Aug;262(2):721-8. | |||||
| REF 147 | WO patent application no. 2006,1317,37, Method and composition for treating inflammatory disorders. | |||||
| REF 148 | Synthesis and antiinflammatory activity of certain 5,6,7,8-tetrahydroquinolines and related compounds. J Med Chem. 1995 Apr 28;38(9):1473-81. | |||||
| REF 149 | CA patent application no. 445634, Acne treatment comprising lipoxygenase inhibitors. | |||||
| REF 150 | Pharmacological nature of nicotine-induced contraction in the rat basilar artery: involvement of arachidonic acid metabolites. Eur J Pharmacol. 2007 Dec 22;577(1-3):109-14. | |||||
| REF 151 | The anti-angiogenic effects of 1-furan-2-yl-3-pyridin-2-yl-propenone are mediated through the suppression of both VEGF production and VEGF-induced ... Vascul Pharmacol. 2009 Mar-Apr;50(3-4):123-31. | |||||
| REF 152 | Cellular oxygenation of 12-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid by 5-lipoxygenase is stimulated by 5-lipoxygenase-activating protein. J Biol Chem. 1998 Dec 4;273(49):32842-7. | |||||
| REF 153 | Hydroxamic acid inhibitors of 5-lipoxygenase: quantitative structure-activity relationships. J Med Chem. 1990 Mar;33(3):992-8. | |||||
| REF 154 | Anti-inflammatory effects and gastrointestinal safety of NNU-hdpa, a novel dual COX/5-LOX inhibitor. Eur J Pharmacol. 2009 Jun 2;611(1-3):100-6. | |||||
| REF 155 | 4-hydroxythiazole inhibitors of 5-lipoxygenase. J Med Chem. 1991 Jul;34(7):2158-65. | |||||
| REF 156 | Indole derivatives as potent inhibitors of 5-lipoxygenase: design, synthesis, biological evaluation, and molecular modeling. Bioorg Med Chem Lett. 2007 May 1;17(9):2414-20. | |||||
| REF 157 | Synthesis and evaluation of benzoxazole derivatives as 5-lipoxygenase inhibitors. Bioorg Med Chem. 2010 Nov 1;18(21):7580-5. | |||||
| REF 158 | Effect of 5-LOX/COX-2 common inhibitor DHDMBF30 on pancreatic cancer cell Capan2. World J Gastroenterol. 2008 Apr 28;14(16):2494-500. | |||||
| REF 159 | Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J Nat Prod. 1999 Sep;62(9):1241-5. | |||||
| REF 160 | Effect of BW B70C, a novel inhibitor of arachidonic acid 5-lipoxygenase, on allergen-induced bronchoconstriction and late-phase lung eosinophil accumulation in sensitised guinea-pigs. Agents Actions.1993 Jan;38(1-2):8-18. | |||||
| REF 161 | Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: effects on bronchial anaphylaxis in anaesthetized guinea-pigs. Br J Pharmacol. 1988 Jun;94(2):540-6. | |||||
| REF 162 | Inhibition of LTB4 biosynthesis in situ by CGS 23885, a potent 5-lipoxygenase inhibitor, correlates with its pleural fluid concentrations in an exp... Naunyn Schmiedebergs Arch Pharmacol. 1997 Apr;355(4):470-4. | |||||
| REF 163 | Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009 Jul 30;124(3):506-12. | |||||
| REF 164 | Cylindol A, a novel biphenyl ether with 5-lipoxygenase inhibitory activity, and a related compound from Imperata Cylindrica. J Nat Prod. 1994 Sep;57(9):1290-3. | |||||
| REF 165 | Studies on 5-lipoxygenase inhibitors. I. Synthesis and 5-lipoxygenase-inhibitory activity of novel hydroxamic acid derivatives. Chem Pharm Bull (Tokyo). 1998 Jun;46(6):966-72. | |||||
| REF 166 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 167 | Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol. 2002 Dec 15;64(12):1767-75. | |||||
| REF 168 | DOI: 10.1517/13543776.2.11.1817 | |||||
| REF 169 | Pharmacology of the dual inhibitor of cyclooxygenase and 5-lipoxygenase 3-hydroxy-5-trifluoromethyl-N-(2-(2-thienyl)-2-phenyl-ethenyl)-benzo (b)thiophene-2-carboxamide. Arzneimittelforschung. 1988 Mar;38(3):372-8. | |||||
| REF 170 | Nonsteroidal anti-inflammatory drugs as scaffolds for the design of 5-lipoxygenase inhibitors. J Med Chem. 1997 Feb 28;40(5):819-24. | |||||
| REF 171 | Pharmacological characterization of SB 202235, a potent and selective 5-lipoxygenase inhibitor: effects in models of allergic asthma. J Pharmacol Exp Ther. 1995 Jun;273(3):1147-55. | |||||
| REF 172 | Induction of apoptosis in blood cells from a patient with acute myelogenous leukemia by SC41661A, a selective inhibitor of 5-lipoxygenase. Prostaglandins Leukot Essent Fatty Acids. 1993 Apr;48(4):323-6. | |||||
| REF 173 | Selective inhibition of 5-lipoxygenase attenuates glomerulonephritis in the rat. Kidney Int. 1994 May;45(5):1301-10. | |||||
| REF 174 | Design of pyrrolo-1,4-benzoxazine derivatives as inhibitors of 5-lipoxygenase and PAF antagonists with anthihistaminic properties, Bioorg. Med. Chem. Lett. 4(20):2383-2388 (1994). | |||||
| REF 175 | Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. FASEB J. 2012 Aug;26(8):3222-9. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

