Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T71266
(Former ID: TTDI02016)
|
|||||
| Target Name |
RAC-gamma serine/threonine-protein kinase (AKT3)
|
|||||
| Synonyms |
STK-2; RAC-PK-gamma; Protein kinase B gamma; Protein kinase Akt-3; PKBG; PKB gamma
Click to Show/Hide
|
|||||
| Gene Name |
AKT3
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| Function |
AKT3 is one of 3 closely related serine/threonine-protein kinases (AKT1, AKT2 and AKT3) called the AKT kinase, and which regulate many processes including metabolism, proliferation, cell survival, growth and angiogenesis. This is mediated through serine and/or threonine phosphorylation of a range of downstream substrates. Over 100 substrate candidates have been reported so far, but for most of them, no isoform specificity has been reported. AKT3 is the least studied AKT isoform. It plays an important role in brain development and is crucial for the viability of malignant glioma cells. AKT3 isoform may also be the key molecule in up-regulation and down-regulation of MMP13 via IL13. Required for the coordination of mitochondrial biogenesis with growth factor-induced increases in cellular energy demands. Down-regulation by RNA interference reduces the expression of the phosphorylated form of BAD, resulting in the induction of caspase-dependent apoptosis.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.11.1
|
|||||
| Sequence |
MSDVTIVKEGWVQKRGEYIKNWRPRYFLLKTDGSFIGYKEKPQDVDLPYPLNNFSVAKCQ
LMKTERPKPNTFIIRCLQWTTVIERTFHVDTPEEREEWTEAIQAVADRLQRQEEERMNCS PTSQIDNIGEEEMDASTTHHKRKTMNDFDYLKLLGKGTFGKVILVREKASGKYYAMKILK KEVIIAKDEVAHTLTESRVLKNTRHPFLTSLKYSFQTKDRLCFVMEYVNGGELFFHLSRE RVFSEDRTRFYGAEIVSALDYLHSGKIVYRDLKLENLMLDKDGHIKITDFGLCKEGITDA ATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILM EDIKFPRTLSSDAKSLLSGLLIKDPNKRLGGGPDDAKEIMRHSFFSGVNWQDVYDKKLVP PFKPQVTSETDTRYFDEEFTAQTITITPPEKYDEDGMDCMDNERRPHFPQFSYSASGRE Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Capivasertib | Drug Info | Approved | Breast cancer | [1] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | ARQ 092 | Drug Info | Phase 2 | Proteus syndrome | [2] | |
| 2 | GSK2141795 | Drug Info | Phase 2 | Lymphoma | [3], [4] | |
| 3 | MK-2206 | Drug Info | Phase 2 | Rectal adenocarcinoma | [5], [6] | |
| 4 | BAY1125976 | Drug Info | Phase 1 | Solid tumour/cancer | [7] | |
| 5 | GSK690693 | Drug Info | Phase 1 | Haematological malignancy | [8], [9] | |
| 6 | LY2780301 | Drug Info | Phase 1 | Solid tumour/cancer | [10] | |
| 7 | LYS-6KAKT1 | Drug Info | Phase 1 | Solid tumour/cancer | [11] | |
| 8 | M2698 | Drug Info | Phase 1 | Solid tumour/cancer | [12] | |
| 9 | SR13668 | Drug Info | Phase 1 | Solid tumour/cancer | [13] | |
| 10 | TCN-P | Drug Info | Phase 1 | Acute myeloid leukaemia | [14] | |
| 11 | XL418 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 11 Modulator drugs | + | ||||
| 1 | Capivasertib | Drug Info | [1] | |||
| 2 | ARQ 092 | Drug Info | [16] | |||
| 3 | GSK2141795 | Drug Info | [17], [18] | |||
| 4 | MK-2206 | Drug Info | [19] | |||
| 5 | BAY1125976 | Drug Info | [16] | |||
| 6 | GSK690693 | Drug Info | [20], [21] | |||
| 7 | LY2780301 | Drug Info | [22] | |||
| 8 | LYS-6KAKT1 | Drug Info | [23] | |||
| 9 | SR13668 | Drug Info | [25], [26] | |||
| 10 | TCN-P | Drug Info | [27] | |||
| 11 | XL418 | Drug Info | [28] | |||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | M2698 | Drug Info | [24] | |||
| 2 | Akt inhibitor VIII | Drug Info | [29] | |||
| 3 | ISC-4 | Drug Info | [30] | |||
| 4 | PMID20005102C1 | Drug Info | [31] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |
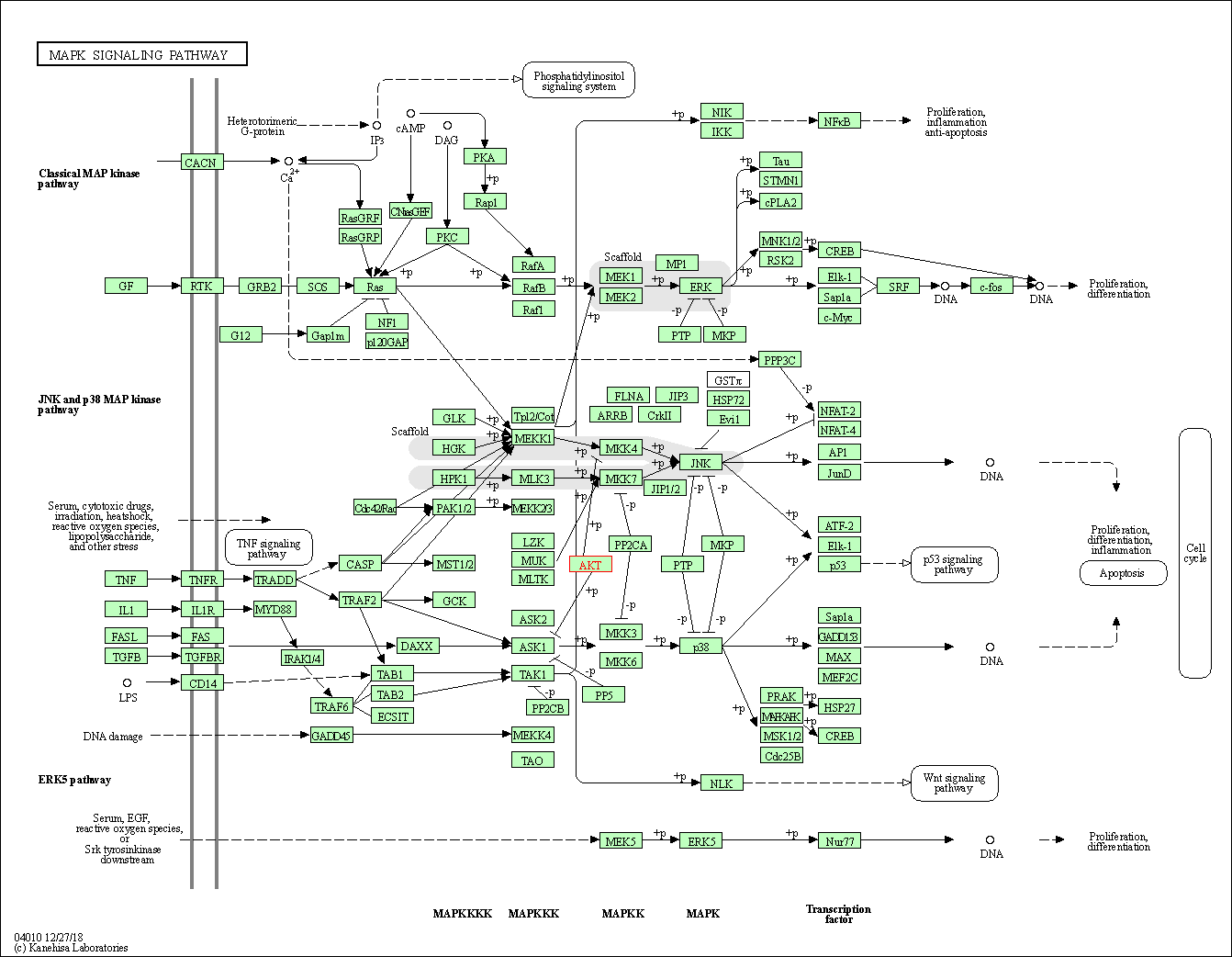
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| ErbB signaling pathway | hsa04012 | Affiliated Target |
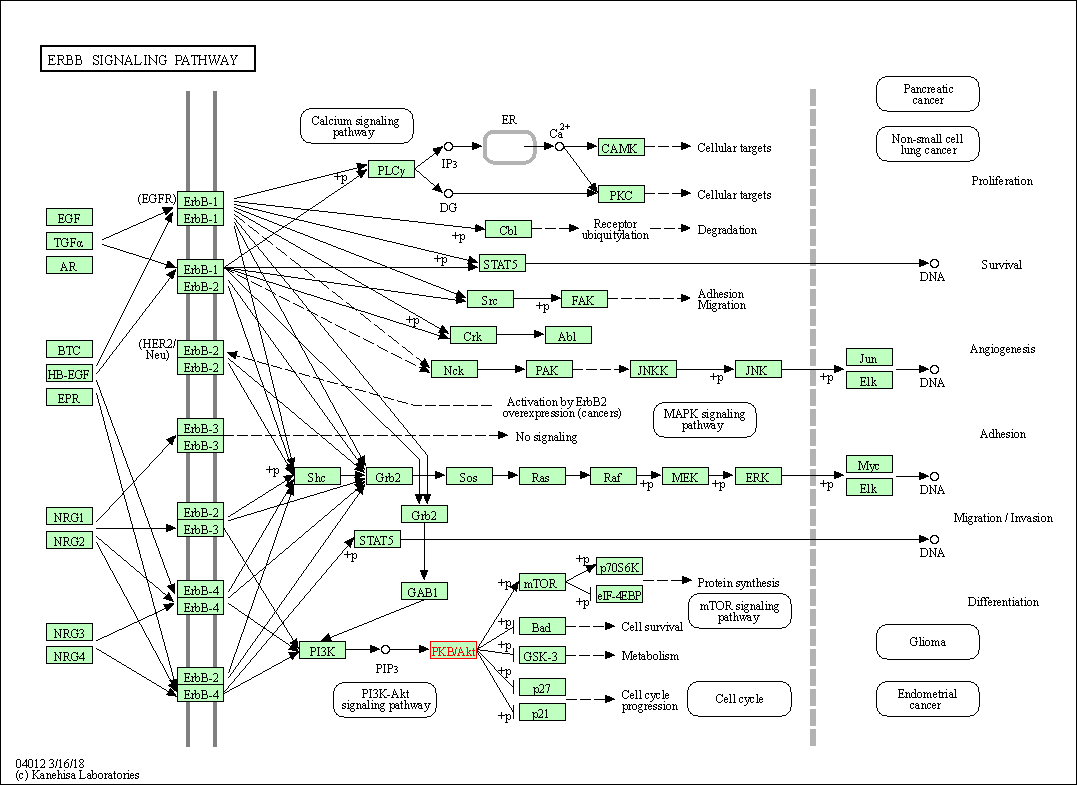
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |
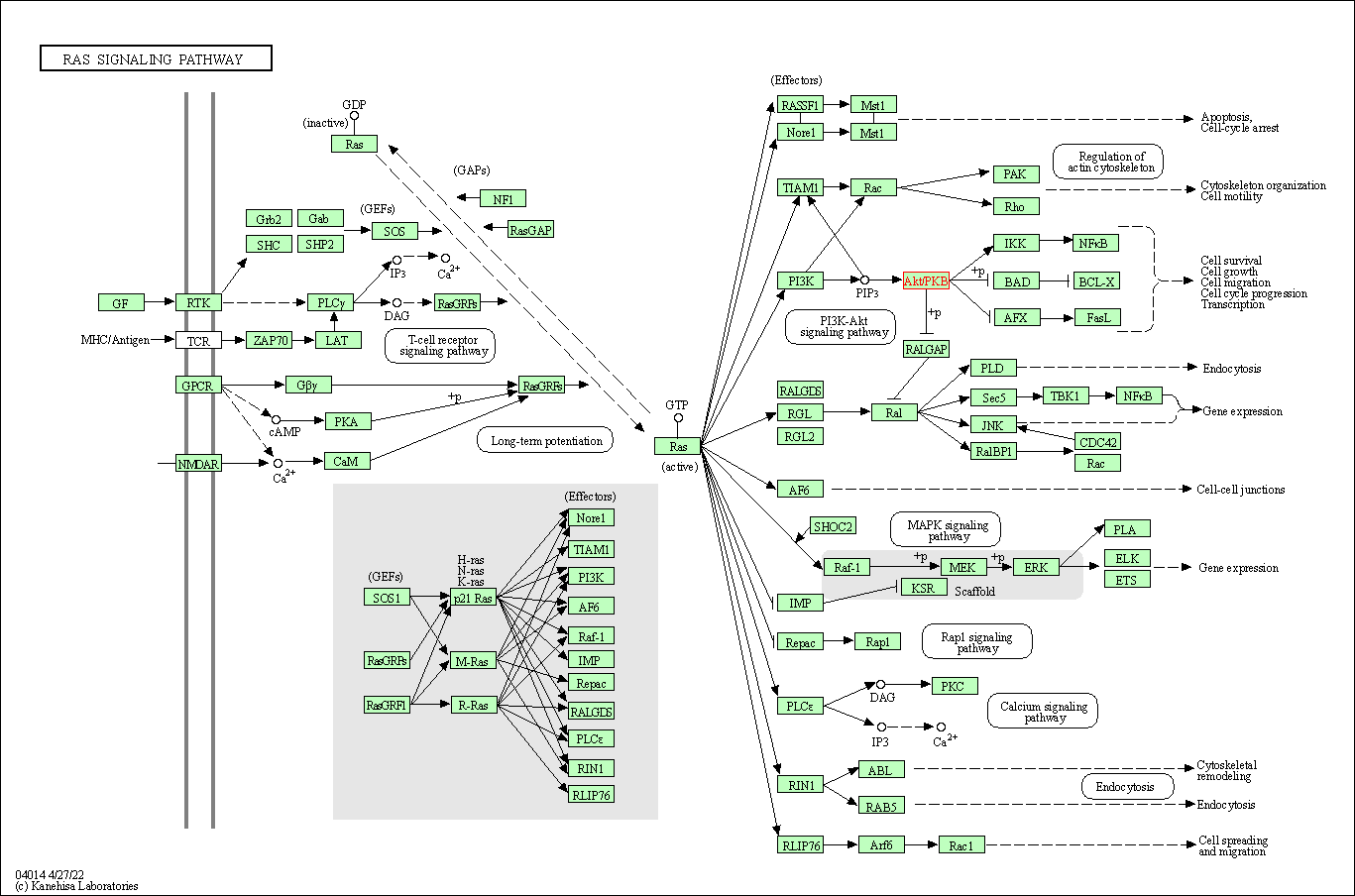
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |
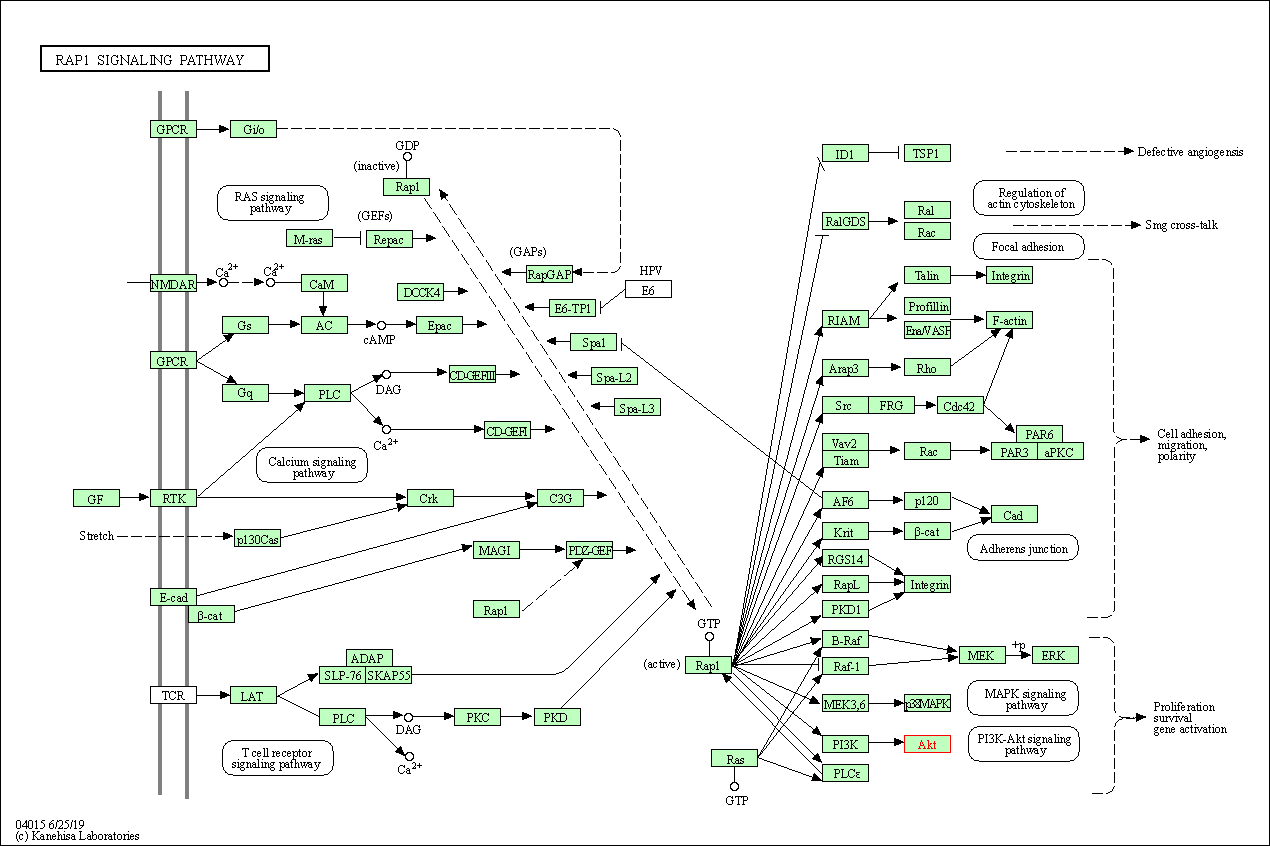
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cAMP signaling pathway | hsa04024 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Chemokine signaling pathway | hsa04062 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| FoxO signaling pathway | hsa04068 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Sphingolipid signaling pathway | hsa04071 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Autophagy - animal | hsa04140 | Affiliated Target |
|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| mTOR signaling pathway | hsa04150 | Affiliated Target |
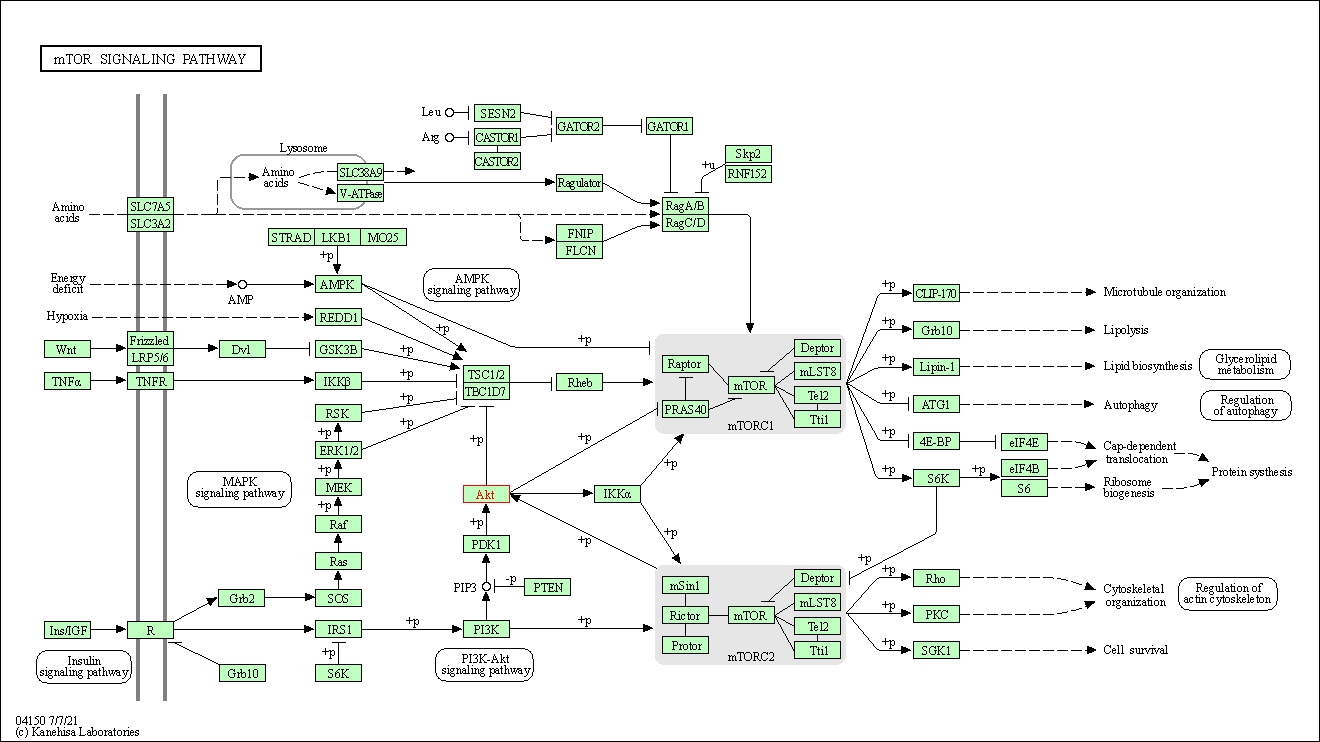
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
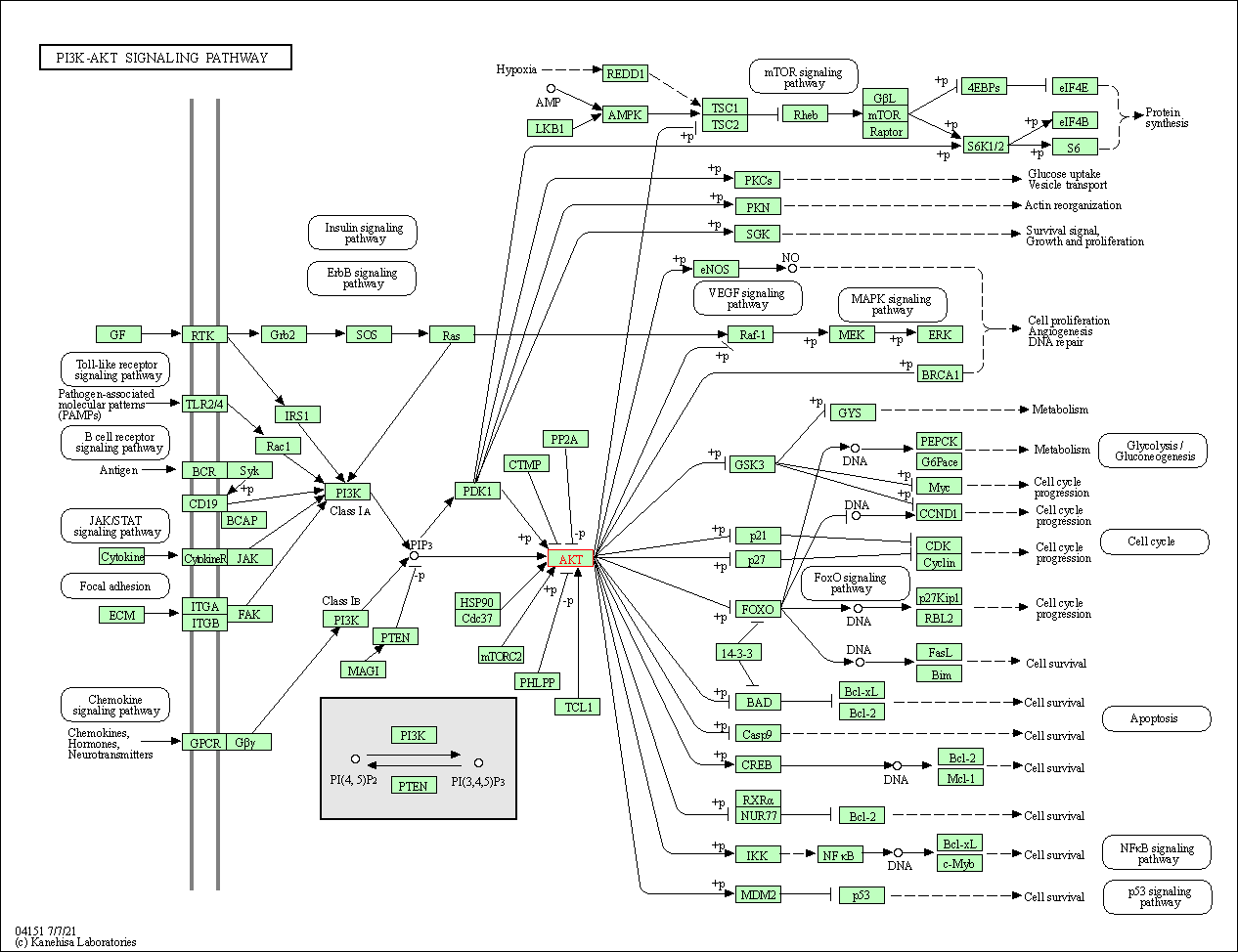
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| AMPK signaling pathway | hsa04152 | Affiliated Target |
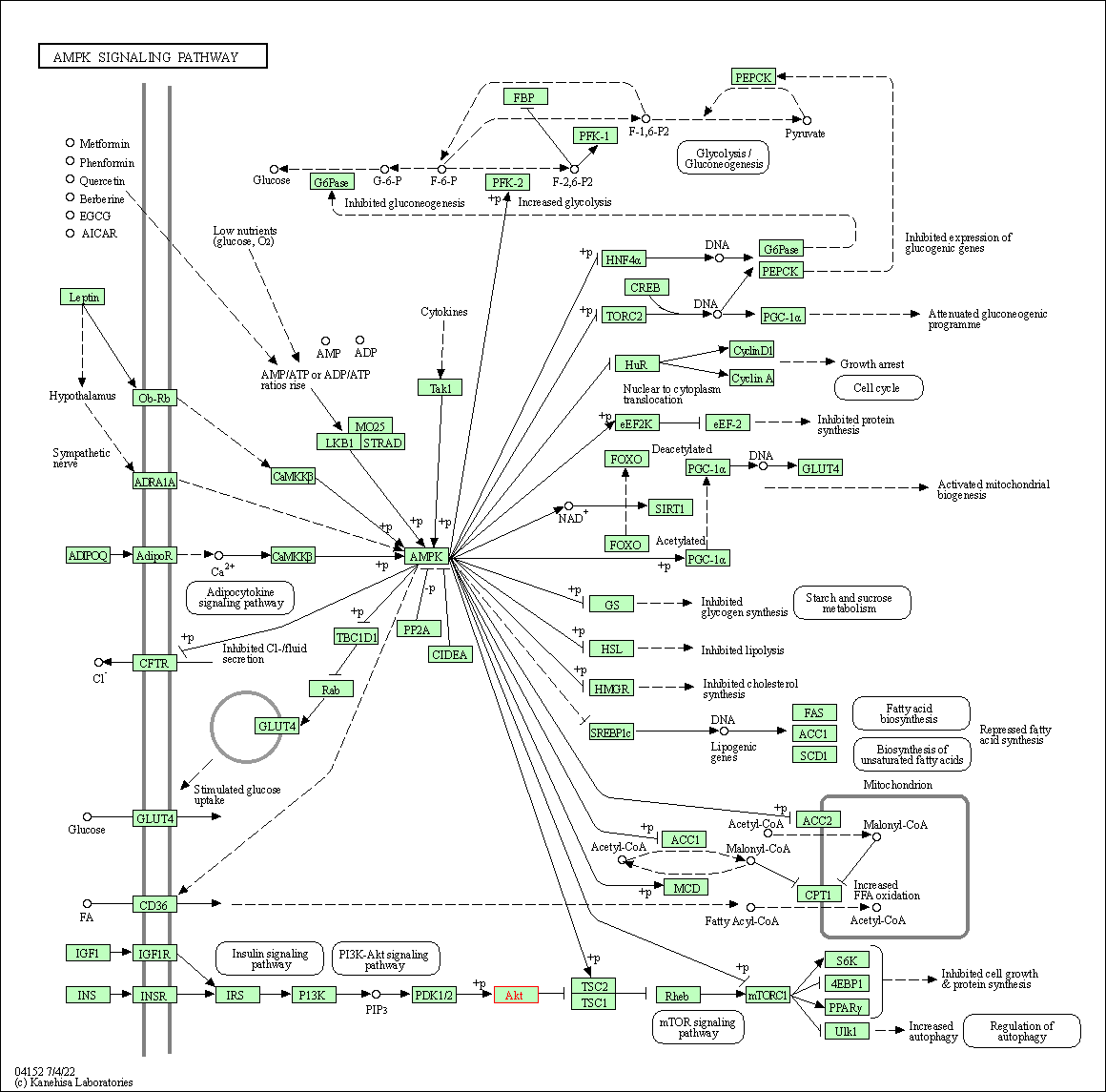
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |
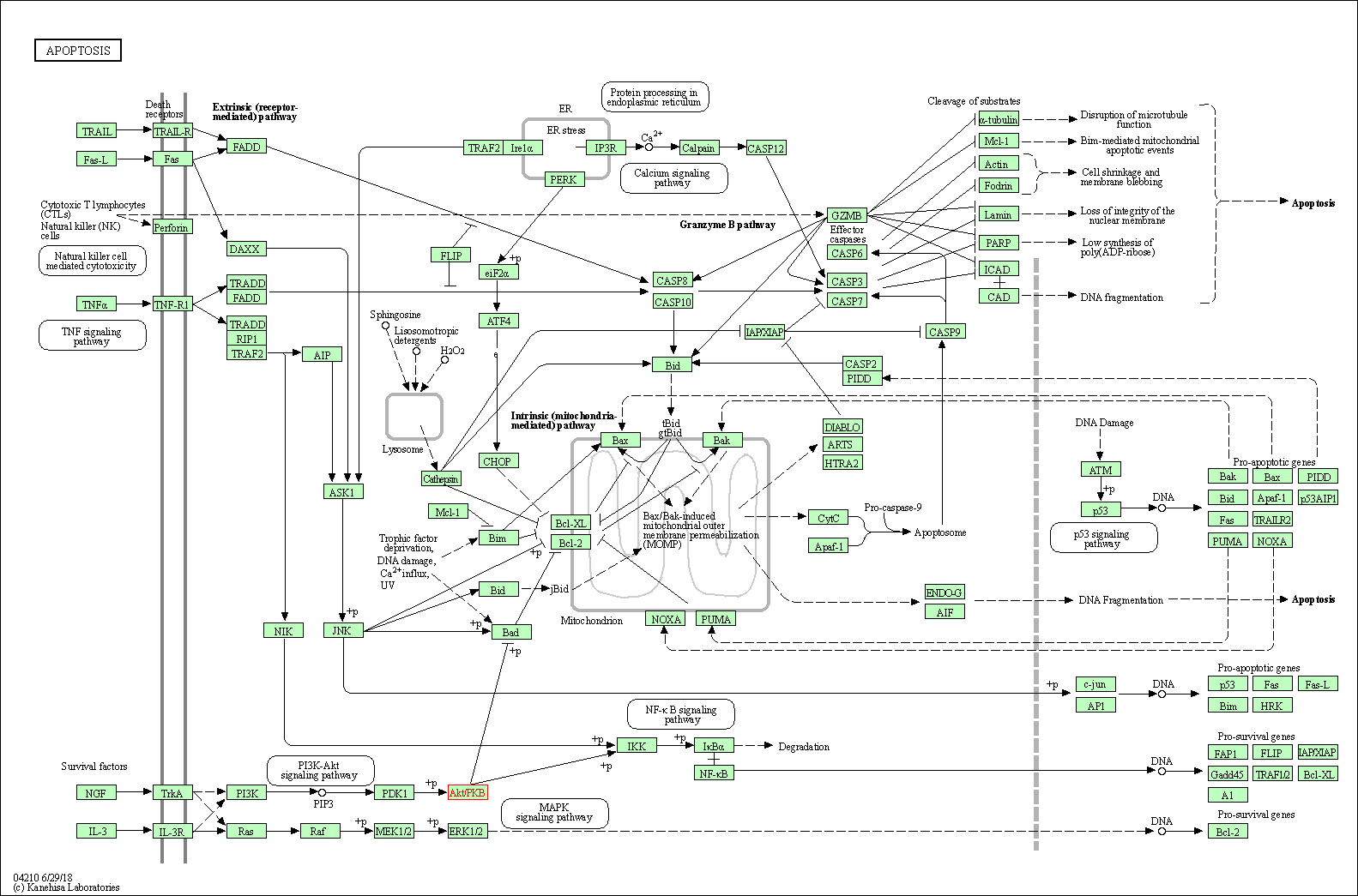
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Longevity regulating pathway | hsa04211 | Affiliated Target |
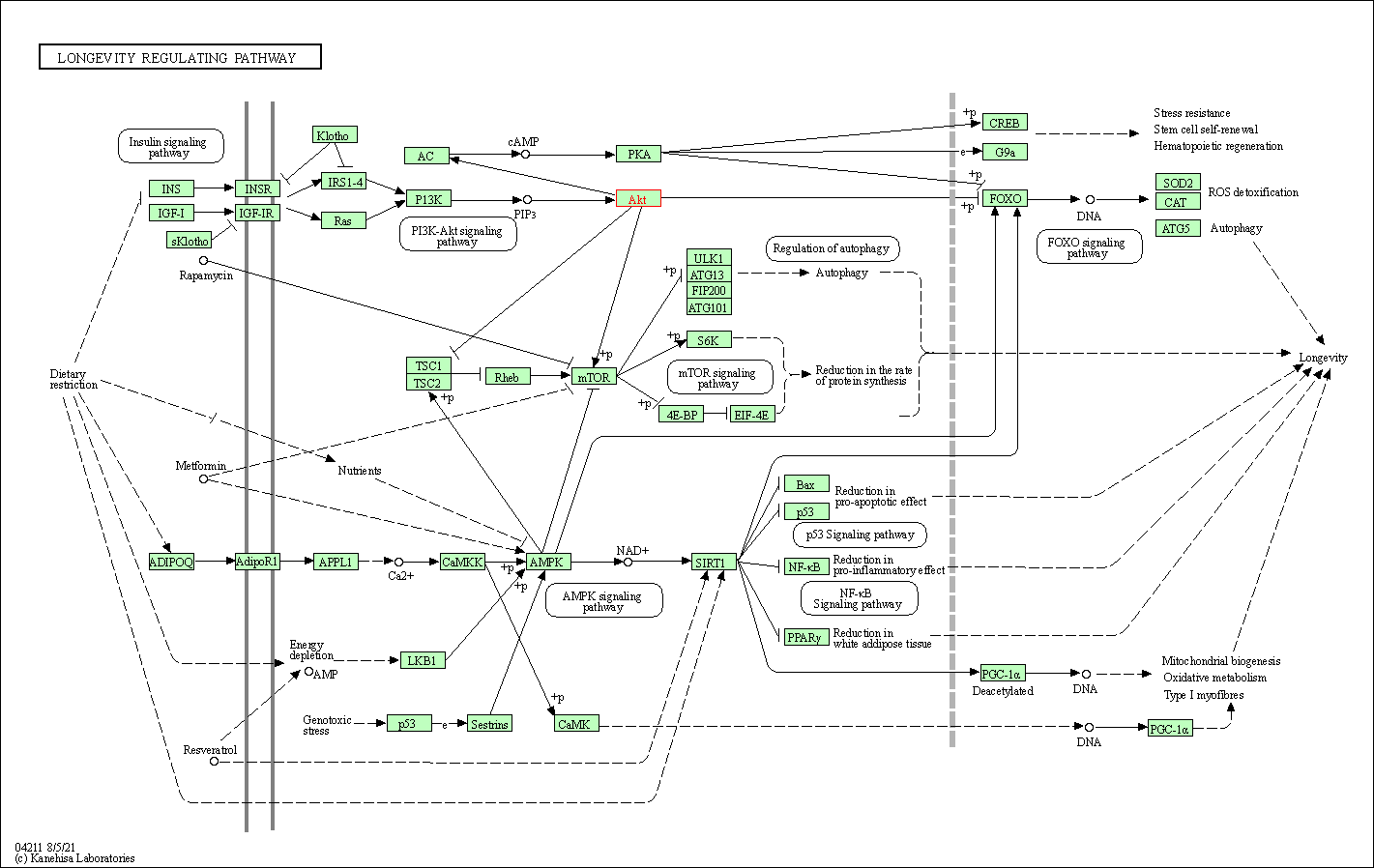
|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Longevity regulating pathway - multiple species | hsa04213 | Affiliated Target |
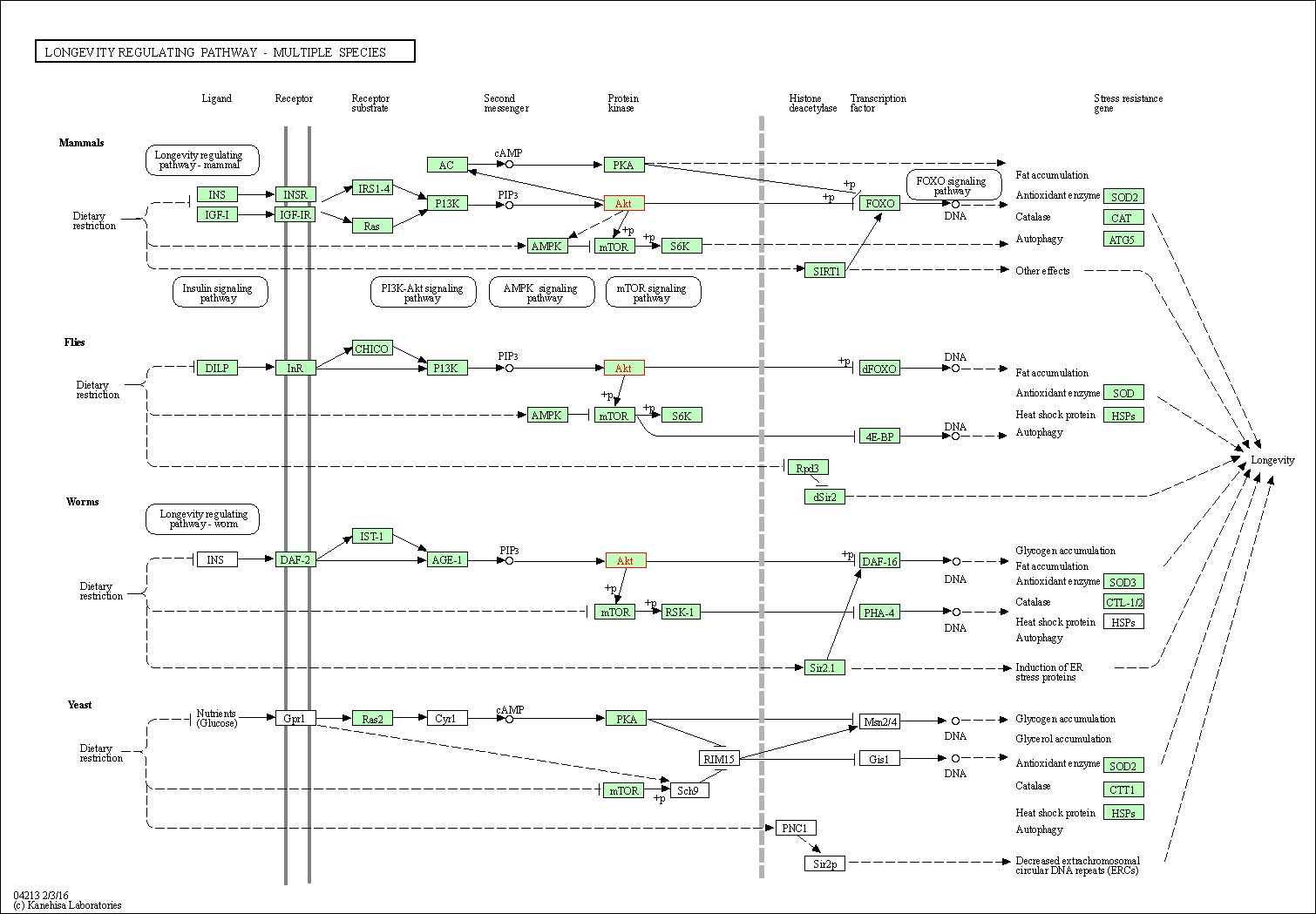
|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Cellular senescence | hsa04218 | Affiliated Target |
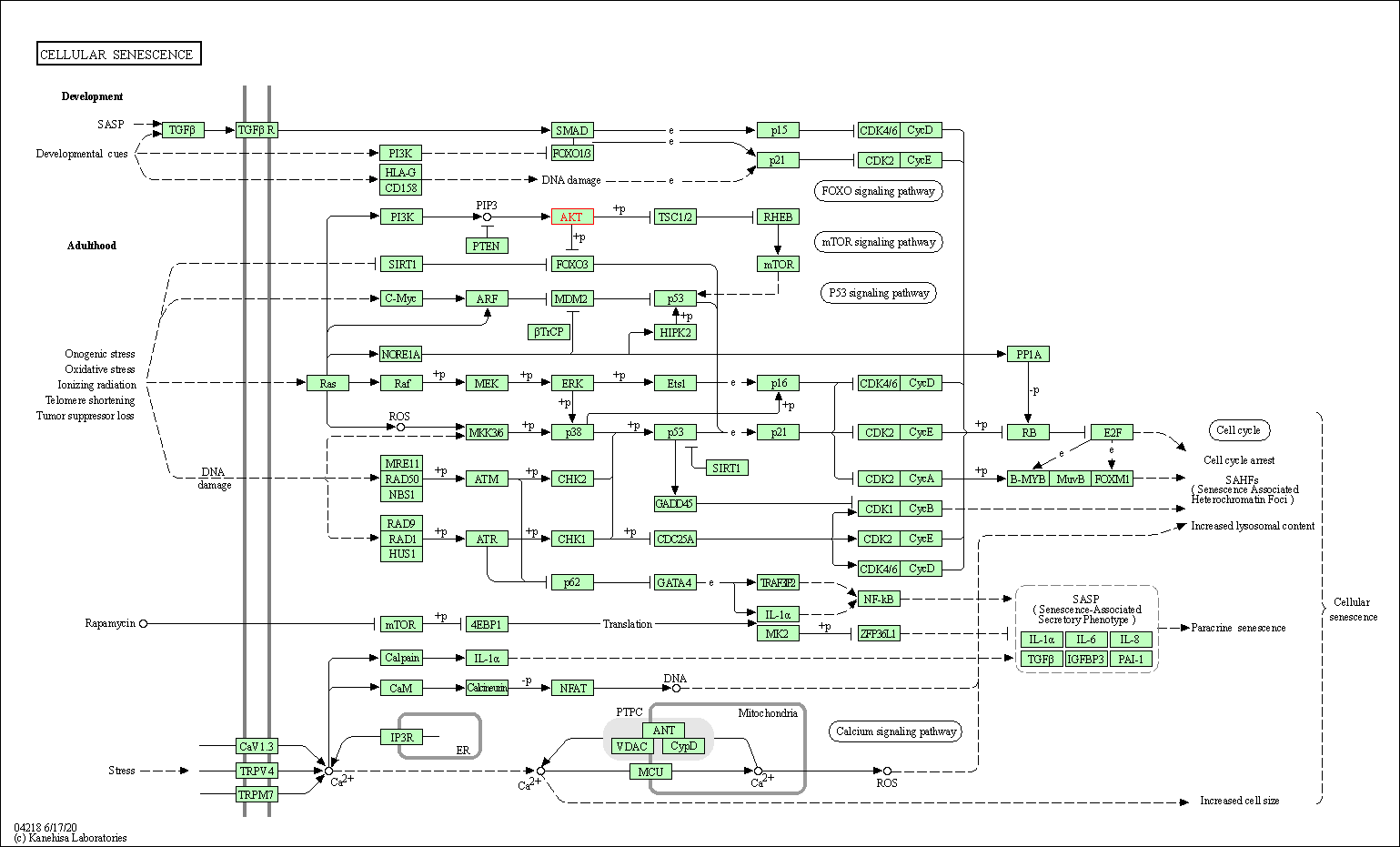
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Adrenergic signaling in cardiomyocytes | hsa04261 | Affiliated Target |
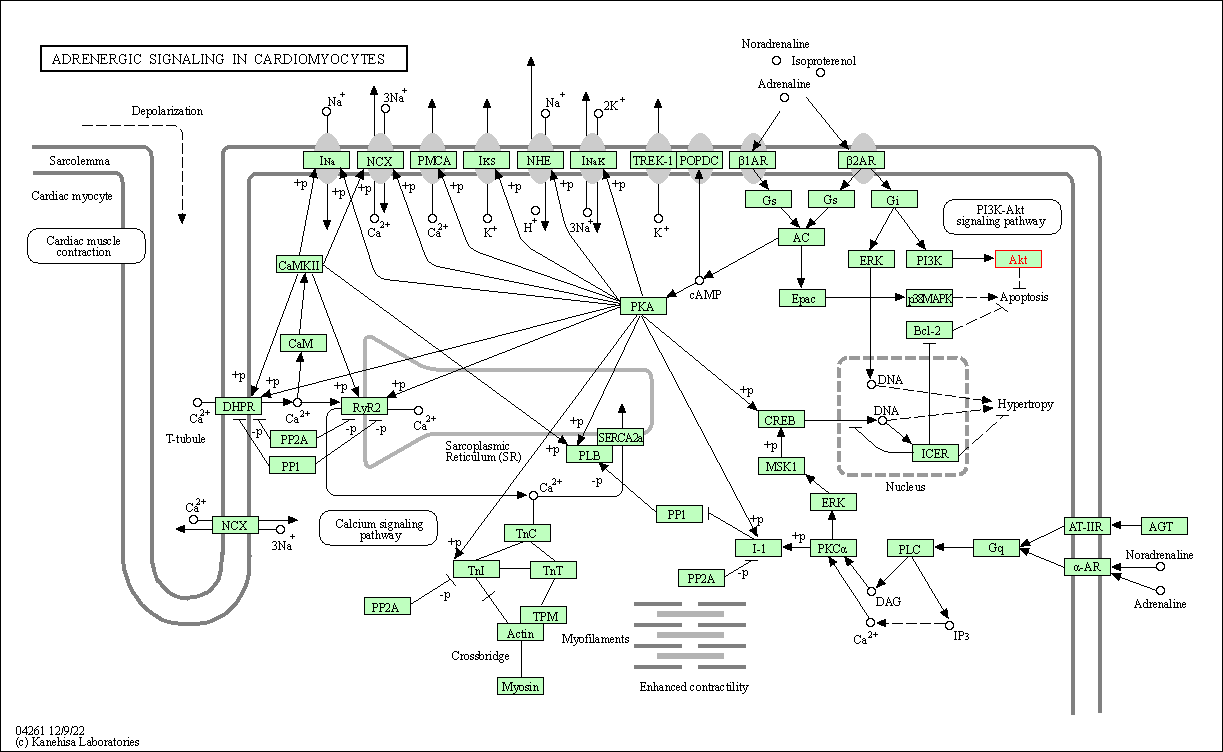
|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| VEGF signaling pathway | hsa04370 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apelin signaling pathway | hsa04371 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |
|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| Focal adhesion | hsa04510 | Affiliated Target |
|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Signaling pathways regulating pluripotency of stem cells | hsa04550 | Affiliated Target |
|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Platelet activation | hsa04611 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| B cell receptor signaling pathway | hsa04662 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Fc gamma R-mediated phagocytosis | hsa04666 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neurotrophin signaling pathway | hsa04722 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Cholinergic synapse | hsa04725 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Dopaminergic synapse | hsa04728 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Regulation of actin cytoskeleton | hsa04810 | Affiliated Target |
|
| Class: Cellular Processes => Cell motility | Pathway Hierarchy | ||
| Insulin signaling pathway | hsa04910 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
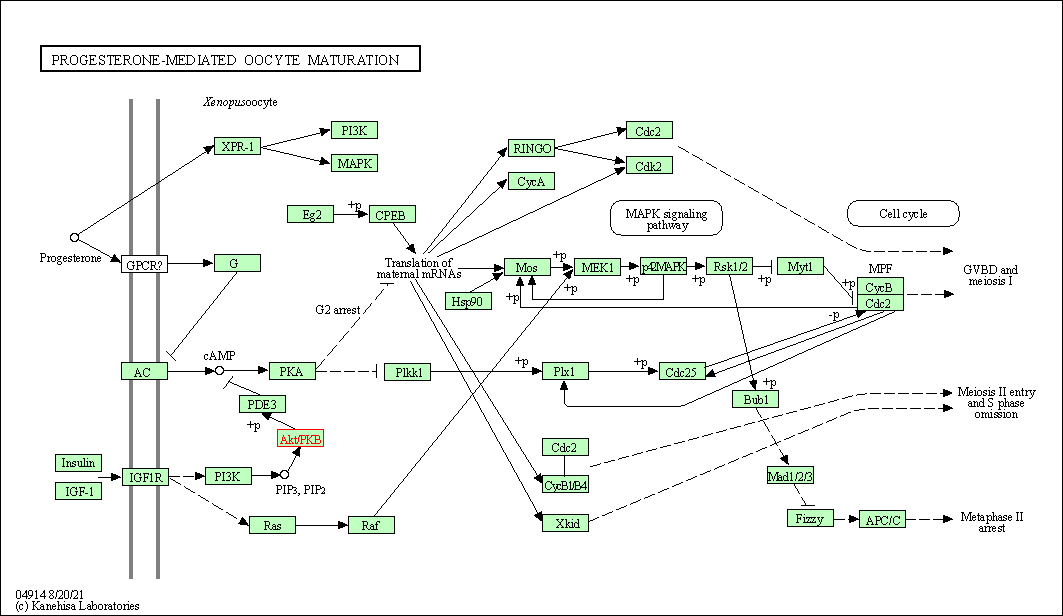
| Progesterone-mediated oocyte maturation | hsa04914 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
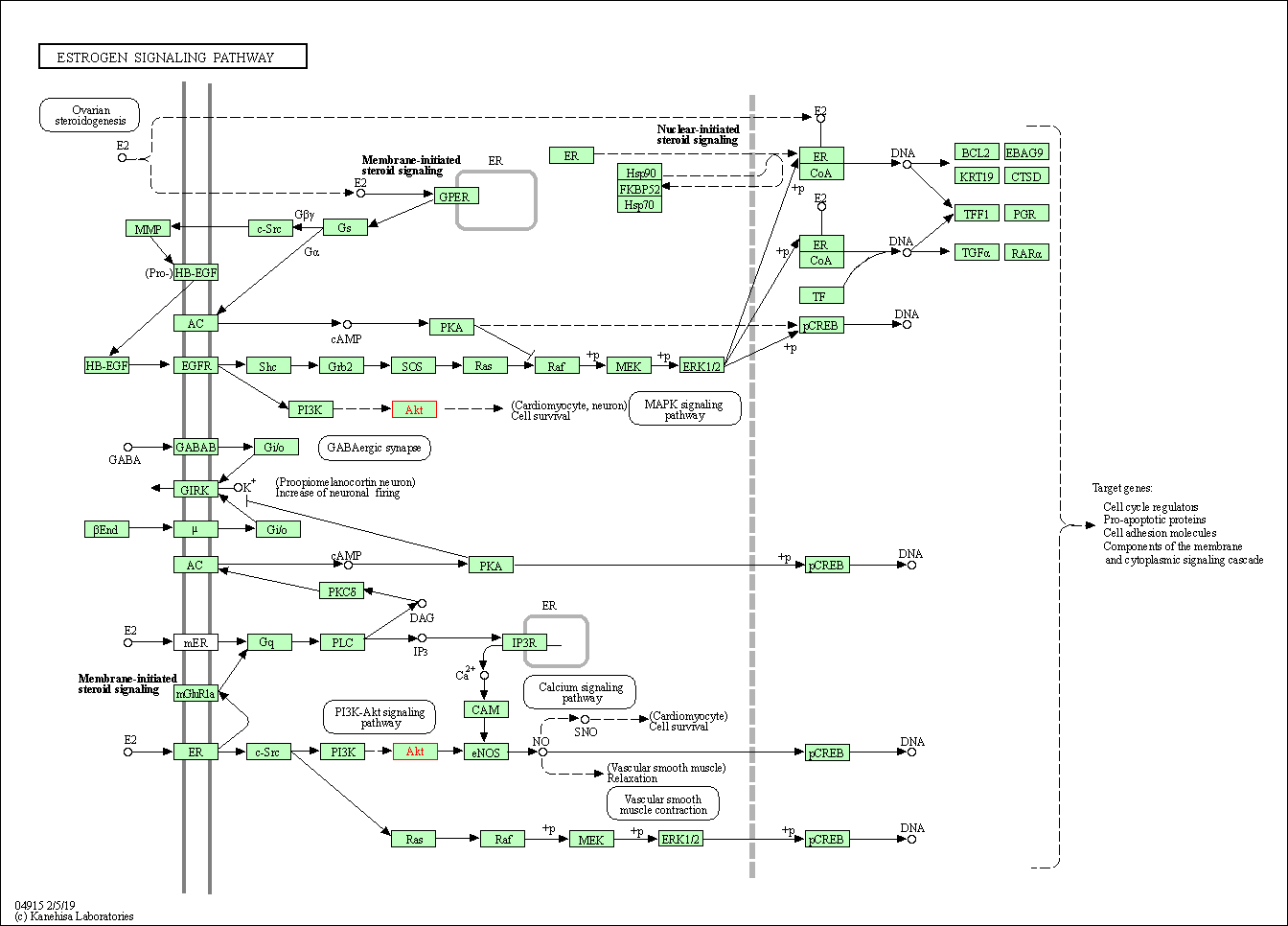
| Estrogen signaling pathway | hsa04915 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
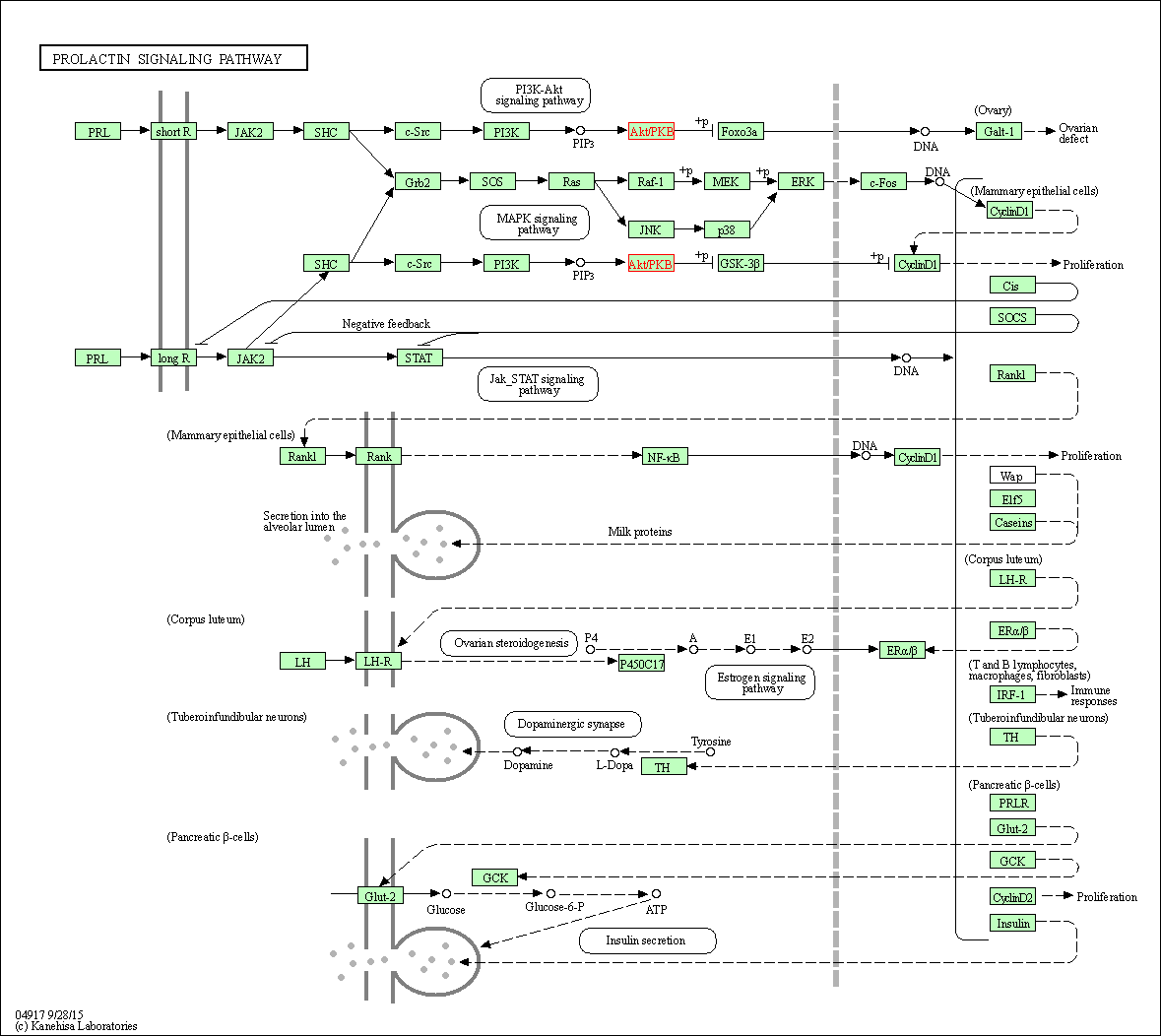
| Prolactin signaling pathway | hsa04917 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
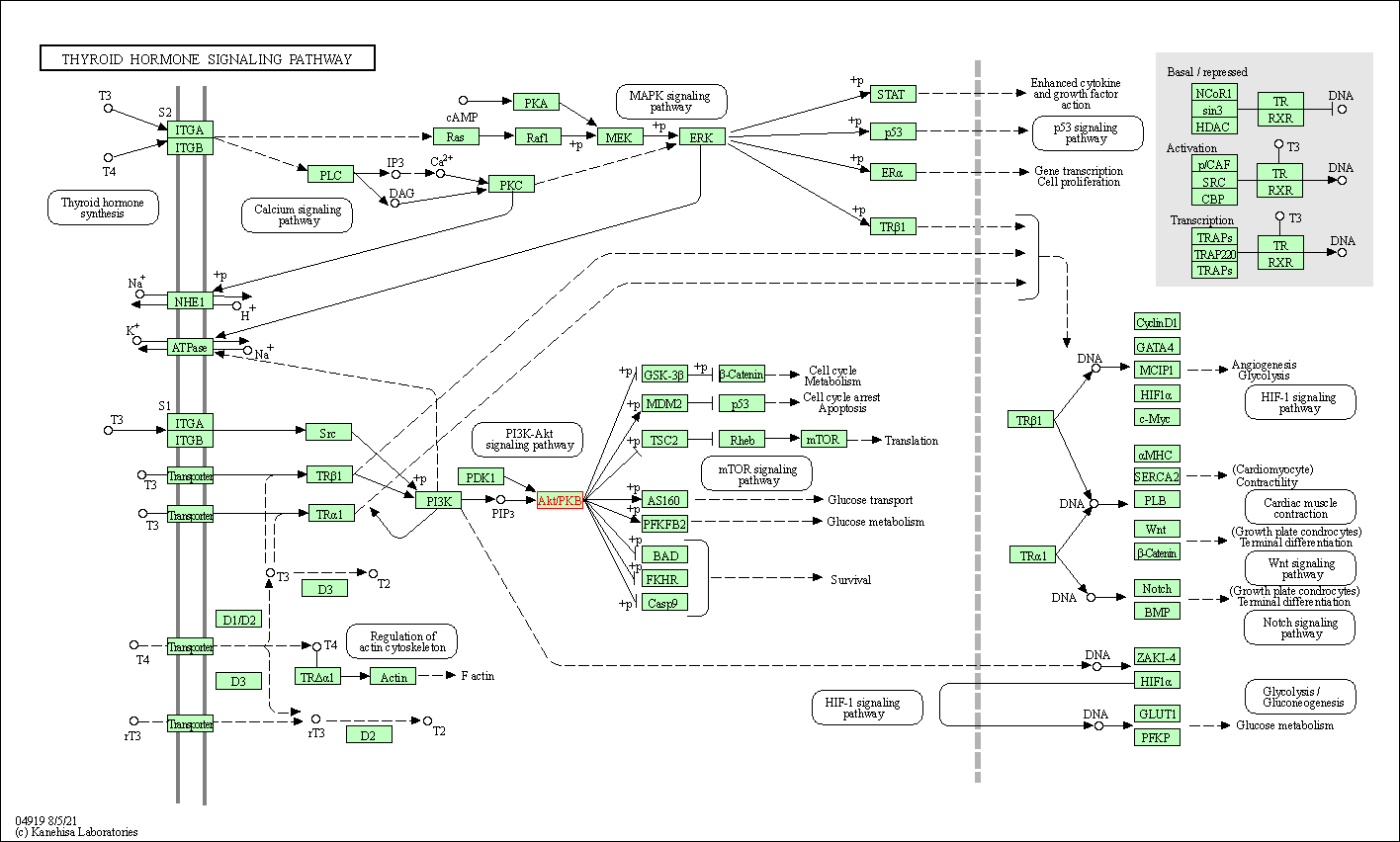
| Thyroid hormone signaling pathway | hsa04919 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Adipocytokine signaling pathway | hsa04920 | Affiliated Target |
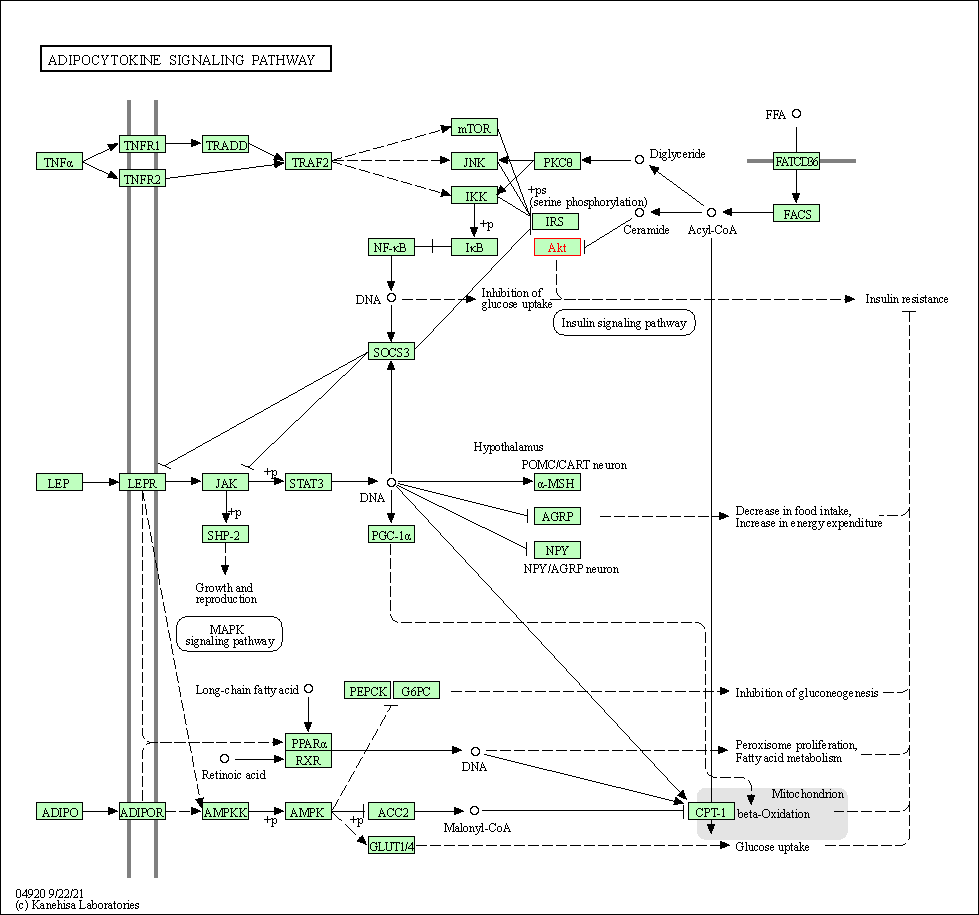
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Glucagon signaling pathway | hsa04922 | Affiliated Target |
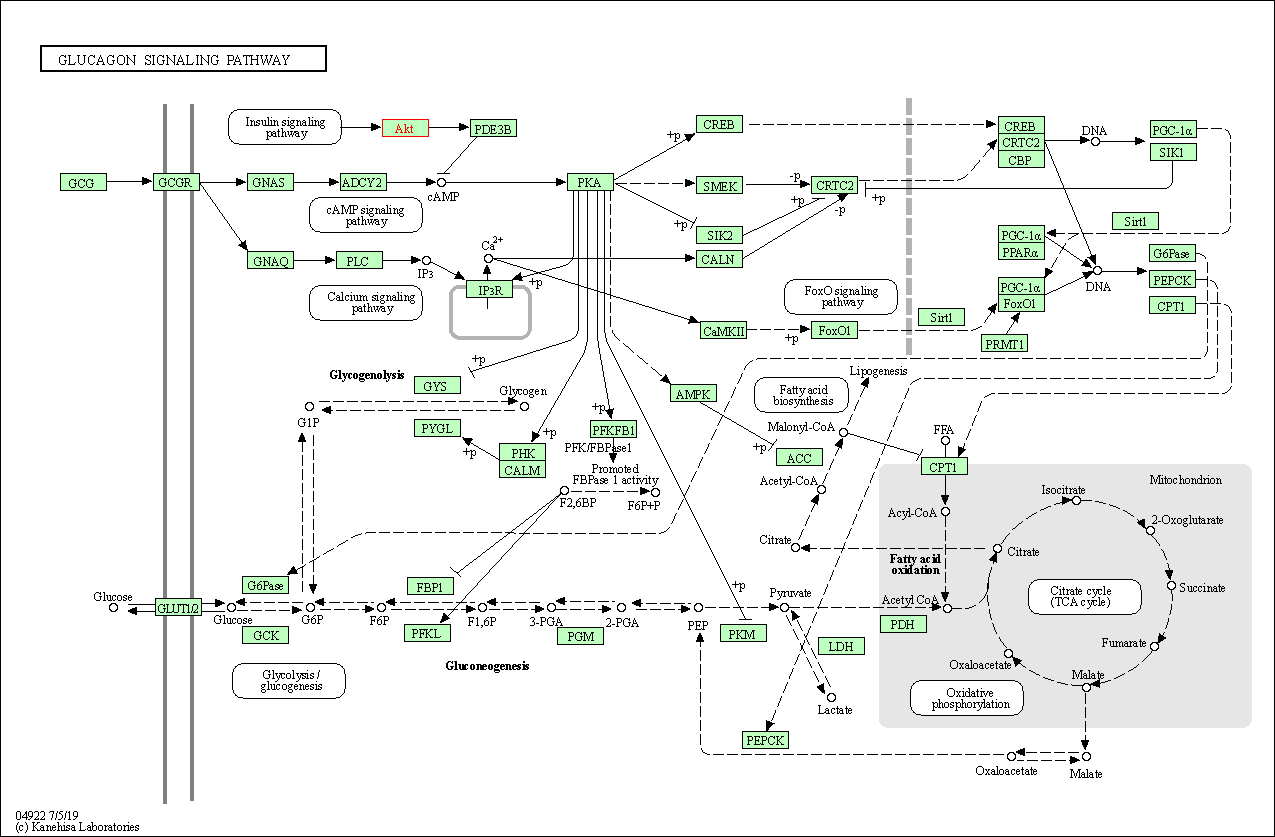
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Regulation of lipolysis in adipocytes | hsa04923 | Affiliated Target |
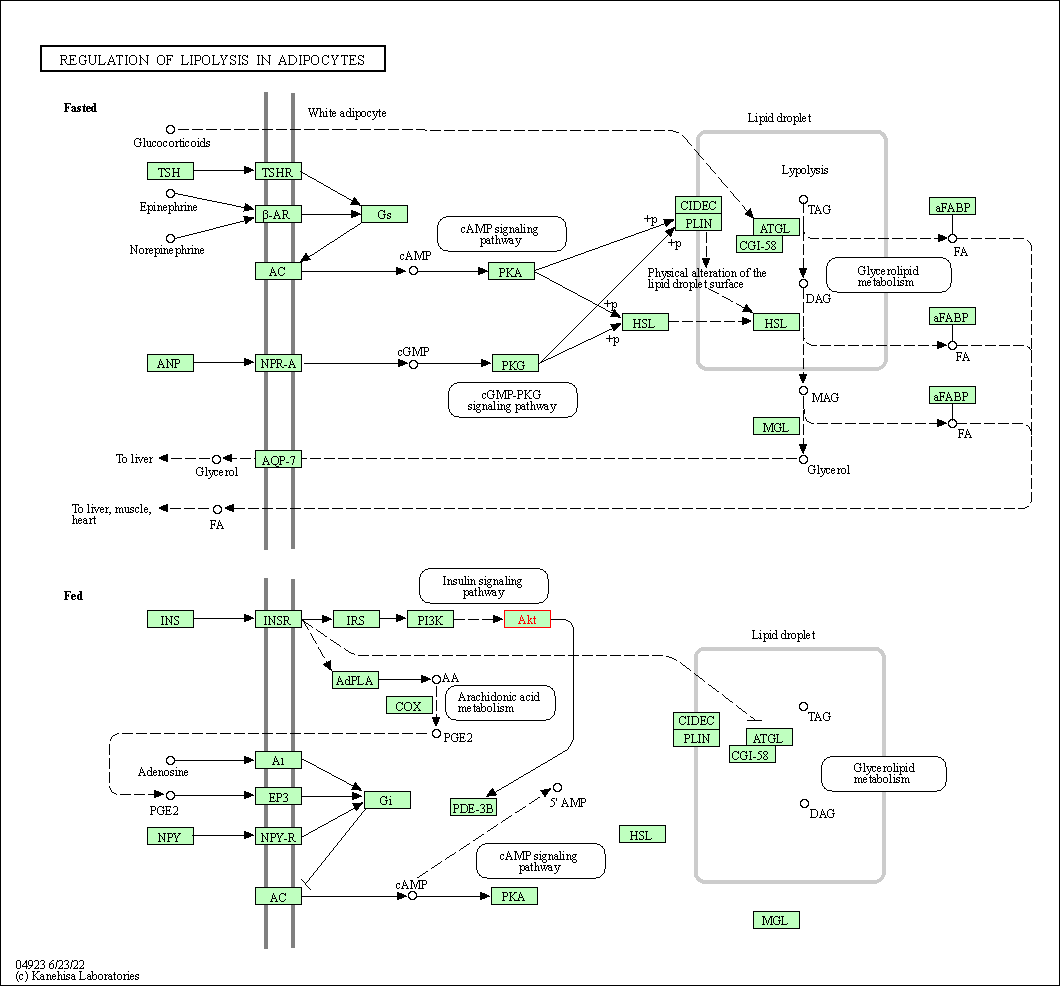
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Relaxin signaling pathway | hsa04926 | Affiliated Target |
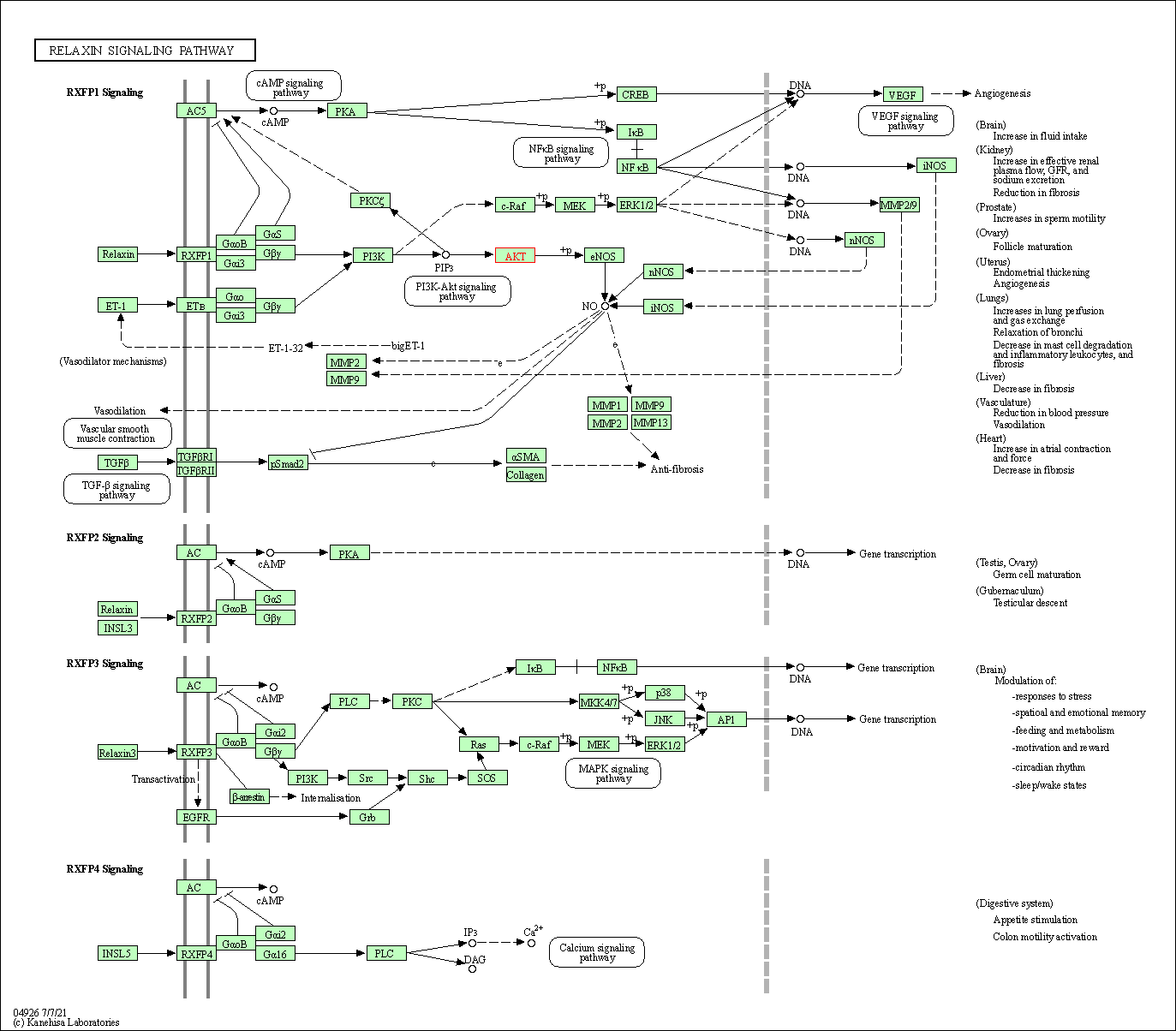
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| GnRH secretion | hsa04929 | Affiliated Target |
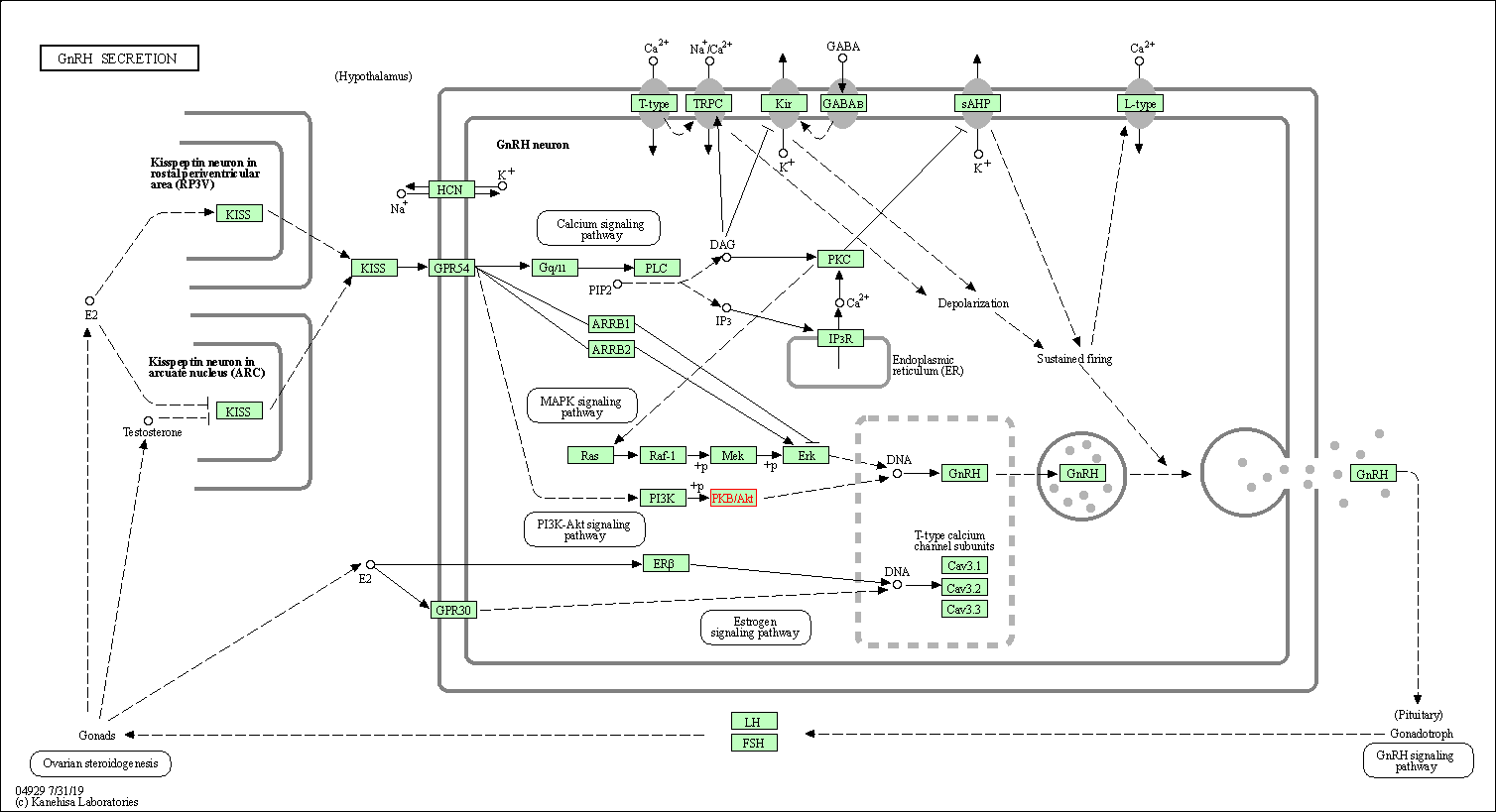
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Growth hormone synthesis, secretion and action | hsa04935 | Affiliated Target |
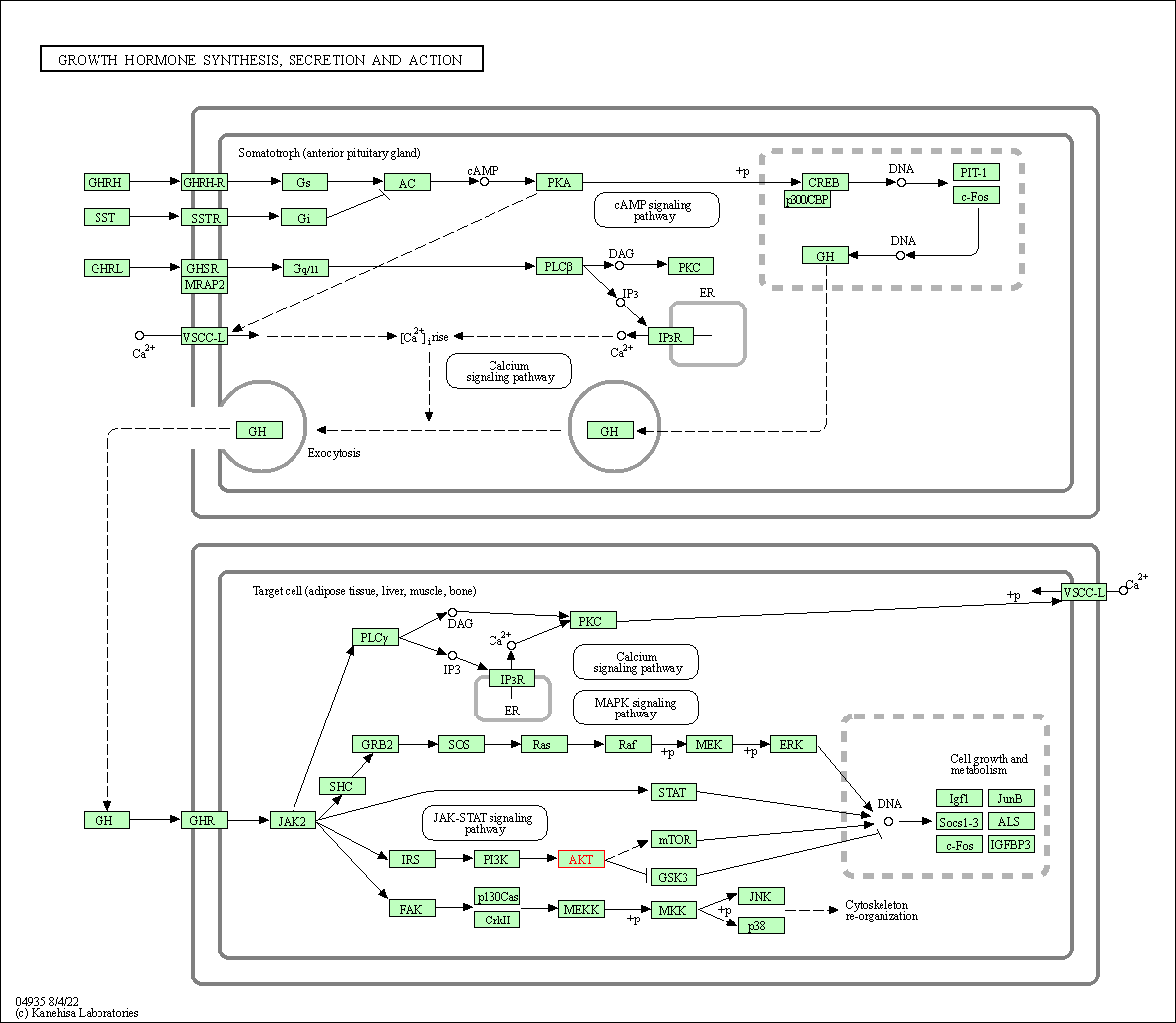
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Carbohydrate digestion and absorption | hsa04973 | Affiliated Target |
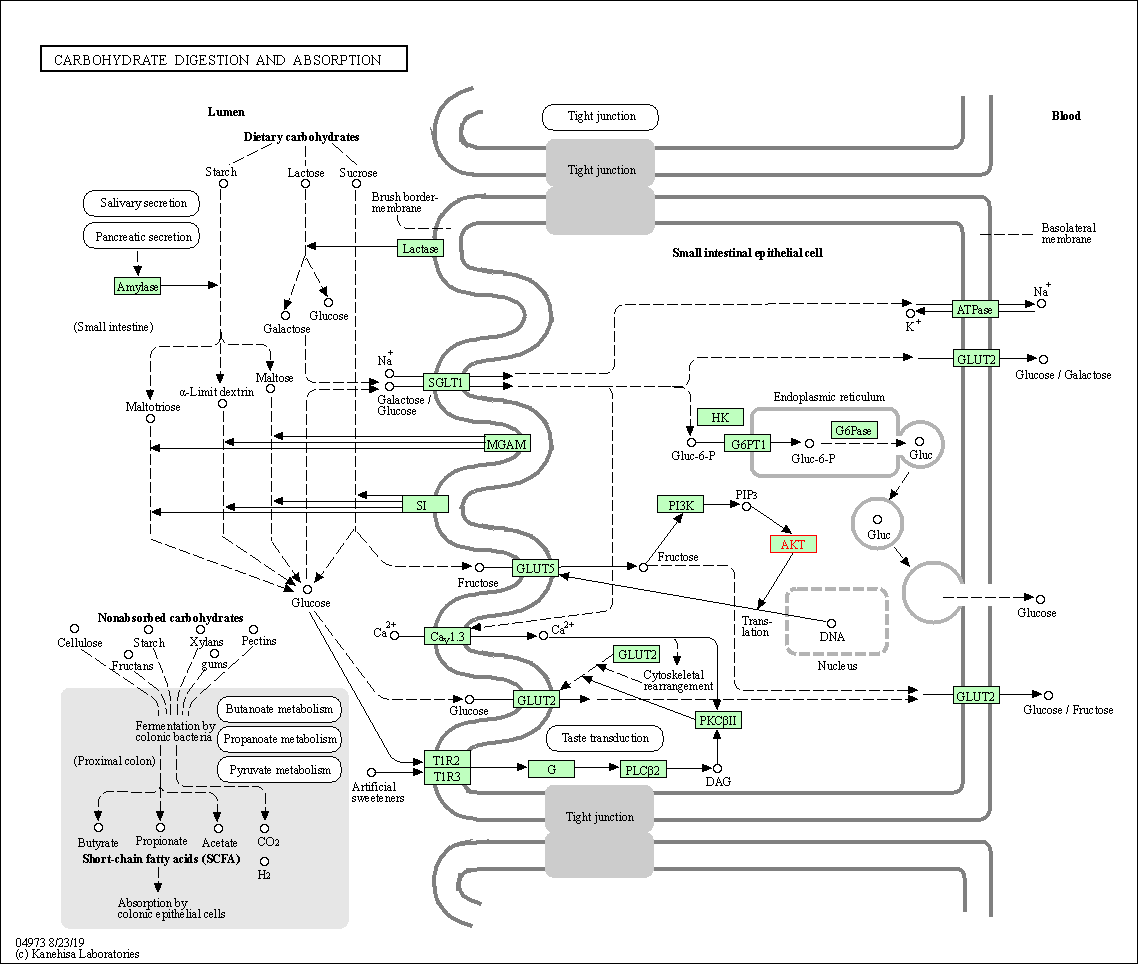
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 12 | Degree centrality | 1.29E-03 | Betweenness centrality | 8.59E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.42E-01 | Radiality | 1.42E+01 | Clustering coefficient | 1.06E-01 |
| Neighborhood connectivity | 5.06E+01 | Topological coefficient | 1.19E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Drug Resistance Mutation (DRM) | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 218197 | |||||
| REF 2 | ClinicalTrials.gov (NCT04316546) ARQ 092 (Miransertib) in Proteus Syndrome. U.S. National Institutes of Health. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7902). | |||||
| REF 4 | ClinicalTrials.gov (NCT01958112) GSK1120212+GSK2141795 for Cervical Cancer. U.S. National Institutes of Health. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7945). | |||||
| REF 6 | ClinicalTrials.gov (NCT01249105) MK-2206 for Recurrent Malignant Glioma. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT01915576) Phase I Dose Escalation Study With an Allosteric AKT 1/2 Inhibitor in Patients. U.S. National Institutes of Health. | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5196). | |||||
| REF 9 | ClinicalTrials.gov (NCT00666081) Study to Investigate AKT Inhibitor GSK690693 in Subjects With Relapsed or Refractory Hematologic Malignancies. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT01115751) A Study in Patients With Advanced or Metastatic Cancer. U.S. National Institutes of Health. | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034531) | |||||
| REF 12 | ClinicalTrials.gov (NCT01971515) First-in-Human Dose Escalation Trial in Subjects With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT00896207) Studying Different Formulations of SR13668 in Healthy Volunteers. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT00363454) Phase I Study of Triciribine Phosphate Monohydrate (TCN-PM, VD-0002) in Adult Subjects With Metastatic Cancer. U.S. National Institutes of Health. | |||||
| REF 15 | ClinicalTrials.gov (NCT00460278) Study of XL418 in Adults With Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 16 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 17 | Inhibiting the akt pathway in cancer treatment: three leading candidates. P T. 2011 Apr;36(4):225-7. | |||||
| REF 18 | Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS One. 2014 Jun 30;9(6):e100880. | |||||
| REF 19 | First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors.J Clin Oncol.2011 Dec 10;29(35):4688-95. | |||||
| REF 20 | Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity.Cancer Res.2008 Apr 1;68(7):2366-74. | |||||
| REF 21 | AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines.Blood.2009 Feb 19;113(8):1723-9. | |||||
| REF 22 | A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drugs. 2015 Jun;33(3):710-9. | |||||
| REF 23 | DOI: 10.1016/S1359-6349(10)71767-5 | |||||
| REF 24 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 25 | Phase 0 clinical chemoprevention trial of the Akt inhibitor SR13668. Cancer Prev Res (Phila). 2011 Mar;4(3):347-53. | |||||
| REF 26 | Pharmacokinetics and enhanced bioavailability of candidate cancer preventative agent, SR13668 in dogs and monkeys. Cancer Chemother Pharmacol. 2010 May;65(6):1109-16. | |||||
| REF 27 | Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT.Invest New Drugs.2011 Dec;29(6):1381-9. | |||||
| REF 28 | Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer | |||||
| REF 29 | Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005 Feb 1;15(3):761-4. | |||||
| REF 30 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2286). | |||||
| REF 31 | 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett. 2010 Jan 15;20(2):679-83. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

