Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T15739
(Former ID: TTDC00118)
|
|||||
| Target Name |
Cellular tumor antigen p53 (TP53)
|
|||||
| Synonyms |
Tumor suppressor p53; Phosphoprotein p53; P53; Antigen NY-CO-13
Click to Show/Hide
|
|||||
| Gene Name |
TP53
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Lip/oral cavity/pharynx neoplasm [ICD-11: 2B6E] | |||||
| 2 | Transplant rejection [ICD-11: NE84] | |||||
| 3 | Ovarian dysfunction [ICD-11: 5A80] | |||||
| Function |
Involved in cell cycle regulation as a trans-activator that acts to negatively regulate cell division by controlling a set of genes required for this process. One of the activated genes is an inhibitor of cyclin-dependent kinases. Apoptosis induction seems to be mediated either by stimulation of BAX and FAS antigen expression, or by repression of Bcl-2 expression. In cooperation with mitochondrial PPIF is involved in activating oxidative stress-induced necrosis; the function is largely independent of transcription. Induces the transcription of long intergenic non-coding RNA p21 (lincRNA-p21) and lincRNA-Mkln1. LincRNA-p21 participates in TP53-dependent transcriptional repression leading to apoptosis and seems to have an effect on cell-cycle regulation. Implicated in Notch signaling cross-over. Prevents CDK7 kinase activity when associated to CAK complex in response to DNA damage, thus stopping cell cycle progression. Isoform 2 enhances the transactivation activity of isoform 1 from some but not all TP53-inducible promoters. Isoform 4 suppresses transactivation activity and impairs growth suppression mediated by isoform 1. Isoform 7 inhibits isoform 1-mediated apoptosis. Regulates the circadian clock by repressing CLOCK-ARNTL/BMAL1-mediated transcriptional activation of PER2. Acts as a tumor suppressor in many tumor types; induces growth arrest or apoptosis depending on the physiological circumstances and cell type.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MEEPQSDPSVEPPLSQETFSDLWKLLPENNVLSPLPSQAMDDLMLSPDDIEQWFTEDPGP
DEAPRMPEAAPPVAPAPAAPTPAAPAPAPSWPLSSSVPSQKTYQGSYGFRLGFLHSGTAK SVTCTYSPALNKMFCQLAKTCPVQLWVDSTPPPGTRVRAMAIYKQSQHMTEVVRRCPHHE RCSDSDGLAPPQHLIRVEGNLRVEYLDDRNTFRHSVVVPYEPPEVGSDCTTIHYNYMCNS SCMGGMNRRPILTIITLEDSSGNLLGRNSFEVRVCACPGRDRRTEEENLRKKGEPHHELP PGSTKRALPNNTSSSPQPKKKPLDGEYFTLQIRGRERFEMFRELNEALELKDAQAGKEPG GSRAHSSHLKSKKGQSTSRHKKLMFKTEGPDSD Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A02521 ; BADD_A03004 ; BADD_A05675 ; BADD_A06440 ; BADD_A08298 | |||||
| HIT2.0 ID | T82JWE | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 17 Clinical Trial Drugs | + | ||||
| 1 | Contusugene ladenovec | Drug Info | Phase 3 | Oral cancer | [2] | |
| 2 | QPI-1002 | Drug Info | Phase 3 | Renal transplantation | [3] | |
| 3 | Thymoquinone | Drug Info | Phase 2/3 | Polycystic ovarian syndrome | [4] | |
| 4 | Ad-p53 | Drug Info | Phase 2 | Solid tumour/cancer | [5] | |
| 5 | ALT-801 | Drug Info | Phase 2 | Acute myeloid leukaemia | [6] | |
| 6 | APG-115 | Drug Info | Phase 2 | Prolymphocytic leukaemia | [7] | |
| 7 | APR-246 | Drug Info | Phase 2 | Ovarian cancer | [8] | |
| 8 | INGN-225 | Drug Info | Phase 2 | Head and neck cancer | [9] | |
| 9 | Kevetrin | Drug Info | Phase 2 | Ovarian cancer | [8] | |
| 10 | SGT-53 | Drug Info | Phase 2 | Pancreatic cancer | [8] | |
| 11 | ISA-P53-01 | Drug Info | Phase 1/2 | Colorectal cancer | [10] | |
| 12 | CGM097 | Drug Info | Phase 1 | Solid tumour/cancer | [11] | |
| 13 | COTI-2 | Drug Info | Phase 1 | Cervical cancer | [8] | |
| 14 | Dendritic cell vaccine | Drug Info | Phase 1 | Head and neck cancer | [12] | |
| 15 | HDM201 | Drug Info | Phase 1 | Haematological malignancy | [13] | |
| 16 | ONYX-015 | Drug Info | Phase 1 | Head and neck cancer | [14] | |
| 17 | SAR-405838 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | INGN-234 | Drug Info | Discontinued in Phase 2 | Oral cancer | [16] | |
| 2 | Pifithrin-alpha | Drug Info | Terminated | Toxicity | [17] | |
| 3 | TAR-1 | Drug Info | Terminated | Solid tumour/cancer | [18] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Modulator | [+] 7 Modulator drugs | + | ||||
| 1 | Contusugene ladenovec | Drug Info | [1] | |||
| 2 | QPI-1002 | Drug Info | [19] | |||
| 3 | Ad-p53 | Drug Info | [21] | |||
| 4 | CGM097 | Drug Info | [19] | |||
| 5 | COTI-2 | Drug Info | [8] | |||
| 6 | ONYX-015 | Drug Info | [28], [29] | |||
| 7 | SAR-405838 | Drug Info | [30], [31] | |||
| Inhibitor | [+] 7 Inhibitor drugs | + | ||||
| 1 | Thymoquinone | Drug Info | [20] | |||
| 2 | APG-115 | Drug Info | [8] | |||
| 3 | HDM201 | Drug Info | [27] | |||
| 4 | Pifithrin-alpha | Drug Info | [33] | |||
| 5 | 1-(9-ethyl-9H-carbazol-3-yl)-N-methylmethanamine | Drug Info | [35] | |||
| 6 | NU-8231 | Drug Info | [36] | |||
| 7 | NUTLIN-3 | Drug Info | [37] | |||
| Immunomodulator (Immunostimulant) | [+] 1 Immunomodulator (Immunostimulant) drugs | + | ||||
| 1 | ALT-801 | Drug Info | [6] | |||
| Stimulator | [+] 3 Stimulator drugs | + | ||||
| 1 | APR-246 | Drug Info | [22] | |||
| 2 | Kevetrin | Drug Info | [8] | |||
| 3 | SGT-53 | Drug Info | [24] | |||
| Suppressor | [+] 1 Suppressor drugs | + | ||||
| 1 | INGN-234 | Drug Info | [32] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Norleucine | Ligand Info | |||||
| Structure Description | SIRT1/Activator/Substrate Complex | PDB:4ZZJ | ||||
| Method | X-ray diffraction | Resolution | 2.74 Å | Mutation | No | [38] |
| PDB Sequence |
RHKLF
|
|||||
|
|
||||||
| Ligand Name: Adenosine-5-diphosphoribose | Ligand Info | |||||
| Structure Description | Sir2 H116A-deacetylated p53 peptide-3'-o-acetyl ADP ribose | PDB:2H59 | ||||
| Method | X-ray diffraction | Resolution | 1.90 Å | Mutation | Yes | [39] |
| PDB Sequence |
HKKLMF
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Sphingolipid signaling pathway | hsa04071 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cell cycle | hsa04110 | Affiliated Target |
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| p53 signaling pathway | hsa04115 | Affiliated Target |
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Mitophagy - animal | hsa04137 | Affiliated Target |
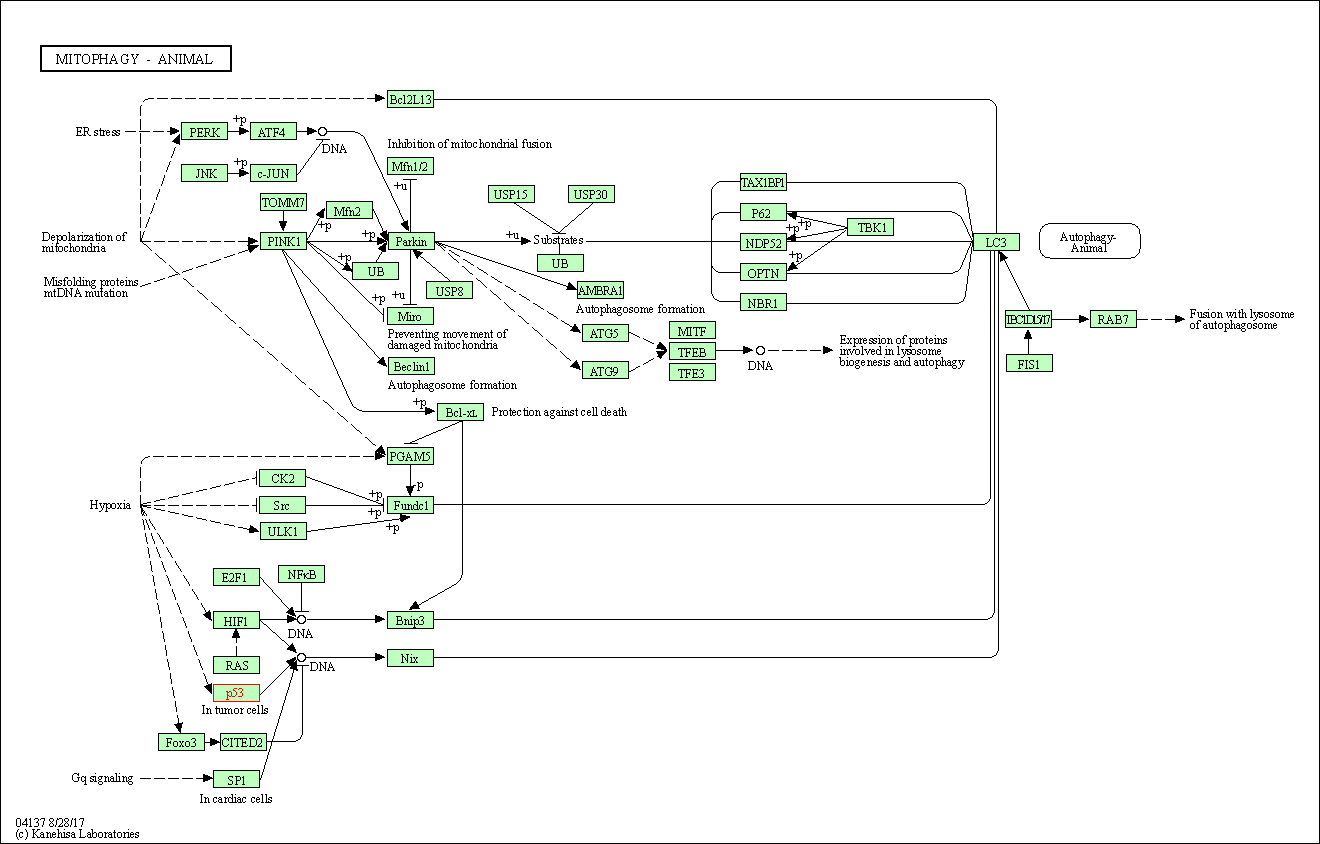
|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
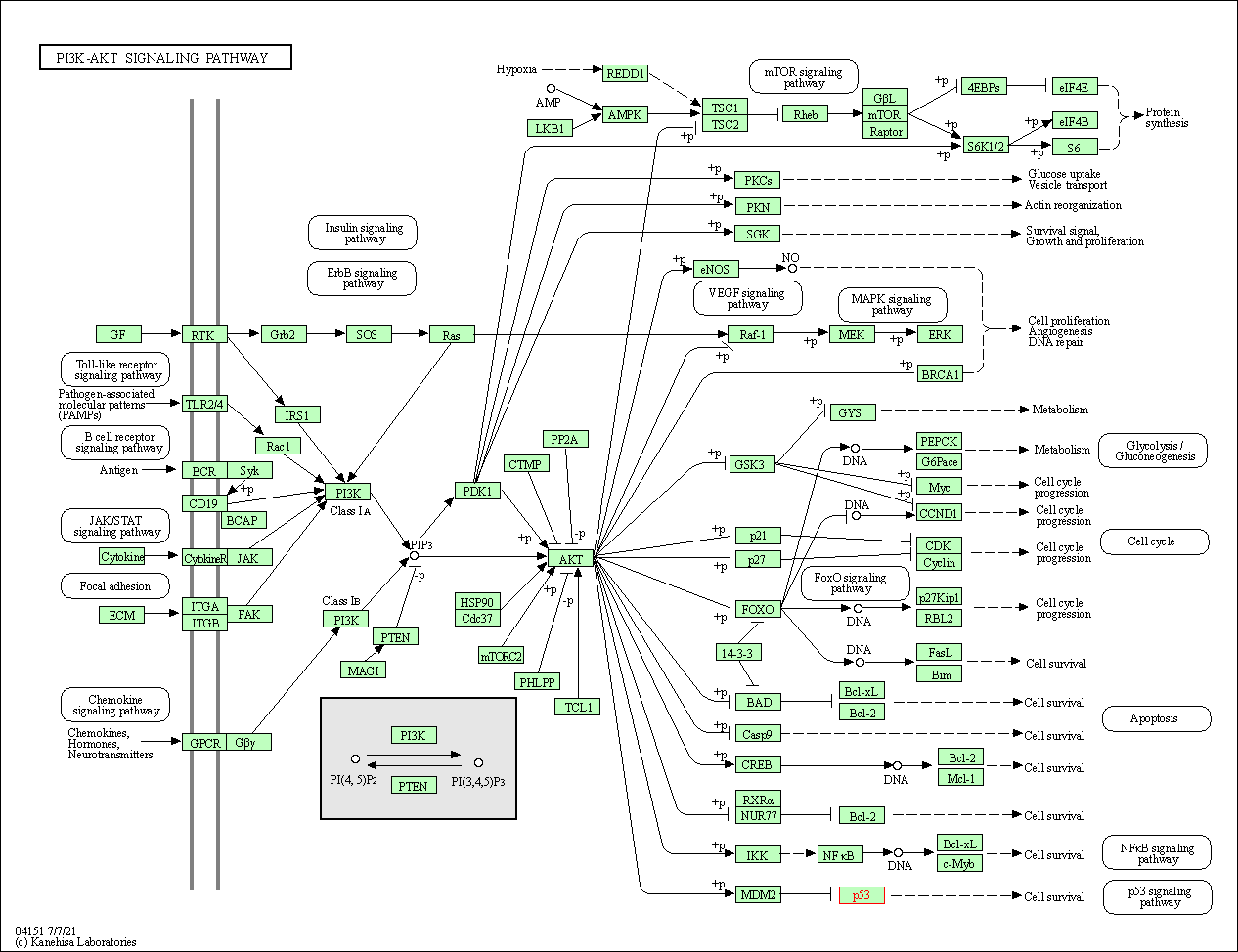
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |
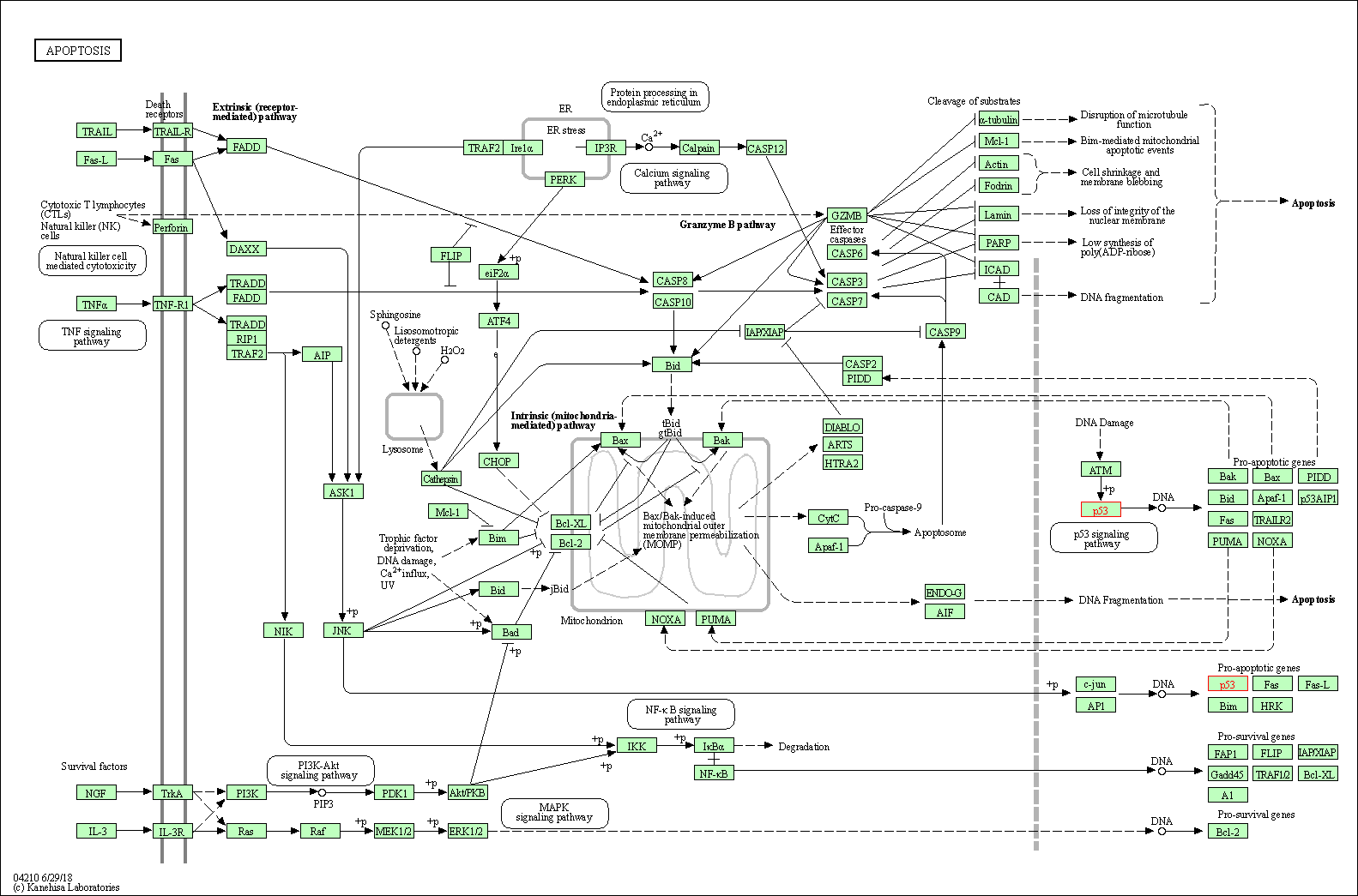
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Longevity regulating pathway | hsa04211 | Affiliated Target |
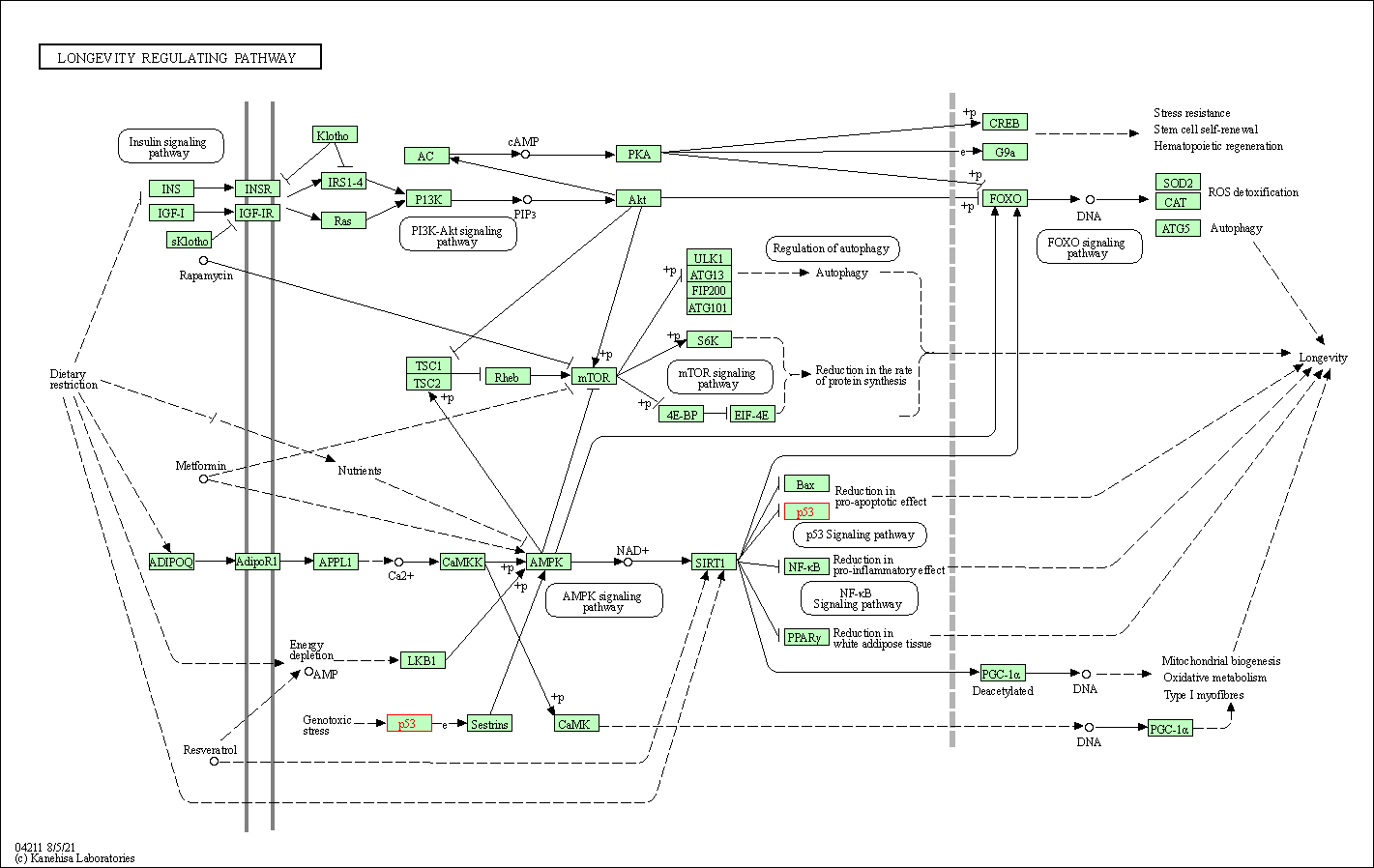
|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Ferroptosis | hsa04216 | Affiliated Target |
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Cellular senescence | hsa04218 | Affiliated Target |
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Wnt signaling pathway | hsa04310 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neurotrophin signaling pathway | hsa04722 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Thyroid hormone signaling pathway | hsa04919 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 264 | Degree centrality | 2.84E-02 | Betweenness centrality | 9.02E-02 |
|---|---|---|---|---|---|
| Closeness centrality | 3.02E-01 | Radiality | 1.50E+01 | Clustering coefficient | 2.59E-02 |
| Neighborhood connectivity | 2.86E+01 | Topological coefficient | 1.16E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Drug Resistance Mutation (DRM) | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | A review of contusugene ladenovec (Advexin) p53 therapy. Curr Opin Mol Ther. 2009 Feb;11(1):54-61. | |||||
| REF 2 | ClinicalTrials.gov (NCT00041613) Study to Compare the Overall Survival of Patients Receiving INGN 201 (Study Drug) With Patients Receiving Methotrexate. U.S. National Institutes of Health. | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | ClinicalTrials.gov (NCT04852510) Amelioration of Polycystic Ovary Syndrome Related Disorders by Supplementation of Thymoquinone and Metformin. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT03544723) Safety and Efficacy of p53 Gene Therapy Combined With Immune Checkpoint Inhibitors in Solid Tumors.. U.S. National Institutes of Health. | |||||
| REF 6 | Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011 Dec 15;17(24):7765-75. | |||||
| REF 7 | ClinicalTrials.gov (NCT04496349) A Study Evaluating APG-115 as a Single Agent or in Combination With APG-2575 in Subjects With T-PLL. U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019096) | |||||
| REF 10 | Clinical pipeline report, company report or official report of ISA Pharmaceuticals BV. | |||||
| REF 11 | ClinicalTrials.gov (NCT01760525) A Phase I Dose Escalation Study of CGM097 in Adult Patients With Selected Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT00404339) Vaccine Therapy in Treating Patients With Head and Neck Cancer. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT02143635) Study to Determine and Evaluate a Safe and Tolerated Dose of HDM201 in Patients With Selected Advanced Tumors That Are TP53wt. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT00006106) ONYX-015 With Cisplatin and Fluorouracil in Treating Patients With Advanced Head and Neck Cancer. U.S. National Institutes of Health. | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036509) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028790) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012866) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031168) | |||||
| REF 19 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 20 | Apoptosis as a mechanism for the treatment of adult T cell leukemia: promising drugs from benchside to bedside. Drug Discov Today. 2020 Jul;25(7):1189-1197. | |||||
| REF 21 | Assessment of p53 gene transfer and biological activities in a clinical study of adenovirus-p53 gene therapy for recurrent ovarian cancer. Cancer Gene Ther. 2003 Mar;10(3):224-38. | |||||
| REF 22 | APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis. 2013 Oct 24;4:e881. | |||||
| REF 23 | INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther. 2010 Jun;10(6):983-91. | |||||
| REF 24 | Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013 Apr;20(4):222-8. | |||||
| REF 25 | WO patent application no. 2013,1850,32, Nanotherapeutics for drug targeting. | |||||
| REF 26 | Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a phase I study. Cancer Immunol Immunother. 2004 Jul;53(7):633-41. | |||||
| REF 27 | National Cancer Institute Drug Dictionary (drug id 761551). | |||||
| REF 28 | Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004 Dec;6(6):611-23. | |||||
| REF 29 | Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000 Nov 15;60(22):6359-66. | |||||
| REF 30 | SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014 Oct 15;74(20):5855-65. | |||||
| REF 31 | SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma.Clin Cancer Res.2016 Mar 1;22(5):1150-60. | |||||
| REF 32 | Prevent Oral Cancer With Mouthwash. Introgen Therapeutics. | |||||
| REF 33 | An evaluation of the ability of pifithrin-alpha and -beta to inhibit p53 function in two wild-type p53 human tumor cell lines. Mol Cancer Ther. 2005 Sep;4(9):1369-77. | |||||
| REF 34 | Regulation of host gene expression by HIV-1 TAR microRNAs. Retrovirology. 2013; 10: 86. | |||||
| REF 35 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 36 | Small-molecule inhibitors of the MDM2-p53 protein-protein interaction based on an isoindolinone scaffold. J Med Chem. 2006 Oct 19;49(21):6209-21. | |||||
| REF 37 | Discovery and optimization of chromenotriazolopyrimidines as potent inhibitors of the mouse double minute 2-tumor protein 53 protein-protein intera... J Med Chem. 2009 Nov 26;52(22):7044-53. | |||||
| REF 38 | Crystallographic structure of a small molecule SIRT1 activator-enzyme complex. Nat Commun. 2015 Jul 2;6:7645. | |||||
| REF 39 | Insights into the sirtuin mechanism from ternary complexes containing NAD+ and acetylated peptide. Structure. 2006 Aug;14(8):1231-40. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

