Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T56365
(Former ID: TTDC00135)
|
|||||
| Target Name |
B-lymphocyte surface antigen B4 (CD19)
|
|||||
| Synonyms |
T-cell surface antigen Leu-12; Leu-12; Differentiation antigen CD19; B-lymphocyte antigen CD19
Click to Show/Hide
|
|||||
| Gene Name |
CD19
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Diffuse large B-cell lymphoma [ICD-11: 2A81] | |||||
| 2 | Mature B-cell lymphoma [ICD-11: 2A85] | |||||
| 3 | Nervous system paraneoplastic/autoimmune disorder [ICD-11: 8E4A] | |||||
| Function |
Decreases the threshold for activation of downstream signaling pathways and for triggering B-cell responses to antigens. Activates signaling pathways that lead to the activation of phosphatidylinositol 3-kinase and the mobilization of intracellular Ca(2+) stores. Is not required for early steps during B cell differentiation in the blood marrow. Required for normal differentiation of B-1 cells. Required for normal B cell differentiation and proliferation in response to antigen challenges. Required for normal levels of serum immunoglobulins, and for production of high-affinity antibodies in response to antigen challenge. Functions as coreceptor for the B-cell antigen receptor complex (BCR) on B-lymphocytes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Immunoglobulin
|
|||||
| UniProt ID | ||||||
| Sequence |
MPPPRLLFFLLFLTPMEVRPEEPLVVKVEEGDNAVLQCLKGTSDGPTQQLTWSRESPLKP
FLKLSLGLPGLGIHMRPLAIWLFIFNVSQQMGGFYLCQPGPPSEKAWQPGWTVNVEGSGE LFRWNVSDLGGLGCGLKNRSSEGPSSPSGKLMSPKLYVWAKDRPEIWEGEPPCLPPRDSL NQSLSQDLTMAPGSTLWLSCGVPPDSVSRGPLSWTHVHPKGPKSLLSLELKDDRPARDMW VMETGLLLPRATAQDAGKYYCHRGNLTMSFHLEITARPVLWHWLLRTGGWKVSAVTLAYL IFCLCSLVGILHLQRALVLRRKRKRMTDPTRRFFKVTPPPGSGPQNQYGNVLSLPTPTSG LGRAQRWAAGLGGTAPSYGNPSSDVQADGALGSRSPPGVGPEEEEGEGYEEPDSEEDSEF YENDSNLGQDQLSQDGSGYENPEDEPLGPEDEDSFSNAESYENEDEELTQPVARTMDFLS PHGSAWDPSREATSLGSQSYEDMRGILYAAPQLRSIRGQPGPNHEEDADSYENMDNPDGP DPAWGGGGRMGTWSTR Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T88WHQ | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 7 Approved Drugs | + | ||||
| 1 | Axicabtagene ciloleucel | Drug Info | Approved | Diffuse large B-cell lymphoma | [2] | |
| 2 | Blinatumomab | Drug Info | Approved | Acute lymphoblastic leukaemia | [3], [4] | |
| 3 | Inebilizumab | Drug Info | Approved | Neuromyelitis optica spectrum disorder | [5] | |
| 4 | Lisocabtagene maraleucel | Drug Info | Approved | Large B-cell lymphoma | [6] | |
| 5 | Loncastuximab tesirine | Drug Info | Approved | Diffuse large B-cell lymphoma | [7] | |
| 6 | Tecartus | Drug Info | Approved | Mantle cell lymphoma | [8] | |
| 7 | Tisagenlecleucel | Drug Info | Approved | Acute lymphoblastic leukaemia | [2] | |
| Clinical Trial Drug(s) | [+] 181 Clinical Trial Drugs | + | ||||
| 1 | Obexelimab | Drug Info | Phase 3 | IgG4 related disease | [9] | |
| 2 | CART-19 | Drug Info | Phase 2/3 | Acute lymphoblastic leukaemia | [10] | |
| 3 | CD19 CAR T cells | Drug Info | Phase 2/3 | Acute lymphoblastic leukaemia | [11] | |
| 4 | MOR-208 | Drug Info | Phase 2/3 | Diffuse large B-cell lymphoma | [12], [13], [14] | |
| 5 | CAR-T cells targeting CD19 | Drug Info | Phase 2 | Non-hodgkin lymphoma | [15] | |
| 6 | CART 19 | Drug Info | Phase 2 | Acute lymphoblastic leukaemia | [16], [17] | |
| 7 | CART-19 | Drug Info | Phase 2 | Non-hodgkin lymphoma | [18] | |
| 8 | CART-19 cells | Drug Info | Phase 2 | Multiple myeloma | [19] | |
| 9 | CD19 CAR T cells | Drug Info | Phase 2 | B-cell chronic lymphocytic leukaemia | [20] | |
| 10 | CD19-targeting CAR T cells | Drug Info | Phase 2 | B-cell lymphoma | [21], [22] | |
| 11 | Coltuximab ravtansine | Drug Info | Phase 2 | Diffuse large B-cell lymphoma | [13] | |
| 12 | CTL019 | Drug Info | Phase 2 | Diffuse large B-cell lymphoma | [23], [24] | |
| 13 | JCAR015 | Drug Info | Phase 2 | B-cell lymphoma | [25] | |
| 14 | KYMRIAH | Drug Info | Phase 2 | Acute lymphoblastic leukaemia | [26] | |
| 15 | MB-CART19.1 | Drug Info | Phase 2 | Precursor B-lymphoblastic neoplasm | [27] | |
| 16 | MDX-1342 | Drug Info | Phase 2 | leukaemia | [28] | |
| 17 | MEDI-551 | Drug Info | Phase 2 | Encephalopathy | [12] | |
| 18 | Minjuvi | Drug Info | Phase 2 | Lymphoma | [29] | |
| 19 | SAR-3419 | Drug Info | Phase 2 | Non-hodgkin lymphoma | [30] | |
| 20 | XLCART001 | Drug Info | Phase 2 | B-cell lymphoma | [31] | |
| 21 | Xmab 5871 | Drug Info | Phase 2 | Autoimmune diabetes | [32] | |
| 22 | 4SCAR19 and 4SCAR123 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 23 | 4SCAR19 and 4SCAR20 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 24 | 4SCAR19 and 4SCAR22 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 25 | 4SCAR19 and 4SCAR30 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 26 | 4SCAR19 and 4SCAR38 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 27 | 4SCAR19 and 4SCAR70 | Drug Info | Phase 1/2 | B-cell lymphoma | [33] | |
| 28 | 4SCAR19 cells | Drug Info | Phase 1/2 | B-cell lymphoma | [34] | |
| 29 | 4SCAR19/22 T cells | Drug Info | Phase 1/2 | B-cell lymphoma | [35] | |
| 30 | ALLO-501A | Drug Info | Phase 1/2 | Large B Cell Lymphoma | [36] | |
| 31 | Anti-CD19 and Anti-CD20 CAR-T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [37] | |
| 32 | Anti-CD19 CAR T cells | Drug Info | Phase 1/2 | B-cell lymphoma | [38], [39] | |
| 33 | Anti-CD19 CAR transduced T cells | Drug Info | Phase 1/2 | Acute lymphocytic leukaemia | [40] | |
| 34 | Anti-CD19 CAR-T | Drug Info | Phase 1/2 | Haematopoietic/lymphoid cancer | [41] | |
| 35 | Anti-CD19 CAR-T cells | Drug Info | Phase 1/2 | Acute lymphocytic leukaemia | [42] | |
| 36 | Anti-CD19 CAR-T cells | Drug Info | Phase 1/2 | leukaemia | [43] | |
| 37 | Anti-CD19 CAR-T cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [44], [45] | |
| 38 | Anti-CD19 CAR-T cells | Drug Info | Phase 1/2 | B-cell chronic lymphocytic leukaemia | [46] | |
| 39 | Anti-CD19-CAR PBL | Drug Info | Phase 1/2 | Mantle cell lymphoma | [47] | |
| 40 | Anti-CD19-CAR vector-transduced T cells | Drug Info | Phase 1/2 | Mantle cell lymphoma | [48], [49] | |
| 41 | Anti-CD19-CAR-T cells | Drug Info | Phase 1/2 | leukaemia | [50] | |
| 42 | Anti-CD19/22-CAR vector-transduced T cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [51] | |
| 43 | Anti-CD20 CAR-T cells | Drug Info | Phase 1/2 | Diffuse large B-cell lymphoma | [52] | |
| 44 | AUTO1 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [53] | |
| 45 | AUTO3 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [54] | |
| 46 | Autologous Anti-CD19CAR-4-1BB-CD3zeta-EGFRt-expressing T Lymphocytes | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [55] | |
| 47 | Autologous CD19-targeting CAR T cells | Drug Info | Phase 1/2 | leukaemia | [56] | |
| 48 | CAR-T cells targeting CD19 | Drug Info | Phase 1/2 | leukaemia | [57] | |
| 49 | CARCIK-CD19 | Drug Info | Phase 1/2 | Acute lymphocytic leukaemia | [58] | |
| 50 | CART-19 cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [59] | |
| 51 | CART-19/22 | Drug Info | Phase 1/2 | leukaemia | [60] | |
| 52 | CART-19/BCMA | Drug Info | Phase 1/2 | Multiple myeloma | [61] | |
| 53 | CD19 and CD20 CAR-T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [62] | |
| 54 | CD19 and CD22 CAR-T Cells | Drug Info | Phase 1/2 | Lymphoma | [63] | |
| 55 | CD19 and CD22 CAR-T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [62] | |
| 56 | CD19 CAR Gene Transduced T Lymphocytes | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [64] | |
| 57 | CD19 CAR T Cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [65] | |
| 58 | CD19 CAR T cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [66] | |
| 59 | CD19 CAR-T Cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [67] | |
| 60 | CD19 CAR-T lymphocytes | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [68] | |
| 61 | CD19 CART | Drug Info | Phase 1/2 | B-cell lymphoma | [69] | |
| 62 | CD19 CART | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [70] | |
| 63 | CD19 specific CAR T cells | Drug Info | Phase 1/2 | Acute leukaemia | [71] | |
| 64 | CD19 targeted chimeric antigen receptor T cells | Drug Info | Phase 1/2 | B-cell lymphoma | [72] | |
| 65 | CD19-CAR T cell | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [73] | |
| 66 | CD19-directed CAR-T cells | Drug Info | Phase 1/2 | leukaemia | [74] | |
| 67 | CD19-targeting CAR T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [75] | |
| 68 | CD19-TCRz-41BB and CD22-TCRz-41BB CAR-T Cells | Drug Info | Phase 1/2 | Haematopoietic/lymphoid cancer | [76] | |
| 69 | CD19.CAR T Cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [77] | |
| 70 | CD19.CAR-T cells | Drug Info | Phase 1/2 | Diffuse large B-cell lymphoma | [78] | |
| 71 | Chimeric Antigen Receptor Modified T cells Targeting CD19 | Drug Info | Phase 1/2 | leukaemia | [79] | |
| 72 | CLIC-1901 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [80] | |
| 73 | Humanized CD19 CAR-T cells | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [81] | |
| 74 | IM19 CAR-T | Drug Info | Phase 1/2 | leukaemia | [82], [83] | |
| 75 | JCAR014 | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [84] | |
| 76 | JCAR017 | Drug Info | Phase 1/2 | Chronic lymphocytic leukaemia | [85], [86] | |
| 77 | KTE-C19 CAR | Drug Info | Phase 1/2 | Diffuse large B-cell lymphoma | [87] | |
| 78 | PBCAR0191 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [88] | |
| 79 | PCAR-019 | Drug Info | Phase 1/2 | Acute lymphocytic leukaemia | [89] | |
| 80 | SCRI-huCAR19v1 | Drug Info | Phase 1/2 | leukaemia | [90] | |
| 81 | SJCAR19 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [91] | |
| 82 | TAK-007 | Drug Info | Phase 1/2 | Hematologic tumour | [92] | |
| 83 | TBI-1501 | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [93] | |
| 84 | TC-110 | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [94] | |
| 85 | TriCAR-T-CD19 | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [95] | |
| 86 | UCART019 | Drug Info | Phase 1/2 | B-cell lymphoma | [96] | |
| 87 | 19-28z T CELLS | Drug Info | Phase 1 | Non-hodgkin lymphoma | [97] | |
| 88 | 4SCAR19 cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [98] | |
| 89 | AFM11 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [99], [13] | |
| 90 | ALLO-501 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [100] | |
| 91 | Allogeneic CART-19 | Drug Info | Phase 1 | leukaemia | [101] | |
| 92 | AMG 562 | Drug Info | Phase 1 | Diffuse large B-cell lymphoma | [102] | |
| 93 | Anti-CD19 anti-CD20 Bispecific CAR-T | Drug Info | Phase 1 | leukaemia | [103] | |
| 94 | Anti-CD19 CAR-T | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [104] | |
| 95 | Anti-CD19 CAR-T cells | Drug Info | Phase 1 | leukaemia | [105] | |
| 96 | Anti-CD19 CART Cells | Drug Info | Phase 1 | Multiple myeloma | [106] | |
| 97 | Anti-CD19 EBV CTL therapy | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [107] | |
| 98 | Anti-CD19-CAR | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [108] | |
| 99 | Anti-CD19-CAR T | Drug Info | Phase 1 | leukaemia | [109] | |
| 100 | Anti-CD19-CAR T cells | Drug Info | Phase 1 | B-cell lymphoma | [110] | |
| 101 | Anti-CD19/BCMA CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [111] | |
| 102 | AntiCD19 CART | Drug Info | Phase 1 | Non-hodgkin lymphoma | [112] | |
| 103 | ARI-0001 cells | Drug Info | Phase 1 | leukaemia | [113] | |
| 104 | BCMA CART and huCART19 | Drug Info | Phase 1 | Multiple myeloma | [114] | |
| 105 | BCMA-CD19 cCAR | Drug Info | Phase 1 | Multiple myeloma | [115] | |
| 106 | C-CAR011 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [116], [117] | |
| 107 | CAR-20/19-T Cells | Drug Info | Phase 1 | B-cell non-hodgkin lymphoma | [118] | |
| 108 | CAR-T cells targeting CD19 | Drug Info | Phase 1 | Lymphoma | [119] | |
| 109 | CAR-T Cells targeting CD19 | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [120] | |
| 110 | CAR19 T cells carrying cytoplasmic activated PD-1 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [121] | |
| 111 | CART-19 | Drug Info | Phase 1 | B-cell lymphoma | [122] | |
| 112 | CART-19 | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [123] | |
| 113 | CART-19 autologous T-cells | Drug Info | Phase 1 | Lymphoma | [124] | |
| 114 | CART-19 cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [125] | |
| 115 | CART-19 cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [126] | |
| 116 | CART-19 T cells | Drug Info | Phase 1 | Multiple myeloma | [127] | |
| 117 | CART-meso-19 T cells | Drug Info | Phase 1 | Pancreatic cancer | [128] | |
| 118 | CART19 cell | Drug Info | Phase 1 | B-cell prolymphocytic leukaemia | [129] | |
| 119 | CART22-65s cells and huCART19 Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [130] | |
| 120 | CARTmeso/19 | Drug Info | Phase 1 | Pancreatic cancer | [131] | |
| 121 | CarVAC | Drug Info | Phase 1 | B-cell lymphoma | [132] | |
| 122 | CC-97540 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [133] | |
| 123 | CD19 CAR T | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [134] | |
| 124 | CD19 CAR T Cells | Drug Info | Phase 1 | Acute lymphocytic leukaemia | [135] | |
| 125 | CD19 CAR T cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [136] | |
| 126 | CD19 CAR T-cells | Drug Info | Phase 1 | Non-hodgkin lymphoma | [137] | |
| 127 | CD19 CAR T-cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [138] | |
| 128 | CD19 CAR-T cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [139] | |
| 129 | CD19 CAR-T cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [140] | |
| 130 | CD19 Redirected Autologous T Cells | Drug Info | Phase 1 | Systemic lupus erythematosus | [141] | |
| 131 | CD19+ CAR T Cells | Drug Info | Phase 1 | leukaemia | [142] | |
| 132 | CD19-CAR and CD28-CAR T Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [143] | |
| 133 | CD19-CAR-T Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [144] | |
| 134 | CD19-CAR-T2 Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [145] | |
| 135 | CD19-directed CAR-T cells | Drug Info | Phase 1 | Non-hodgkin lymphoma | [146] | |
| 136 | CD19-directed CAR-T cells | Drug Info | Phase 1 | leukaemia | [147] | |
| 137 | CD19-targeted CART cells | Drug Info | Phase 1 | leukaemia | [148] | |
| 138 | CD19-TriCAR-T | Drug Info | Phase 1 | Non-hodgkin lymphoma | [149] | |
| 139 | CD19.CAR-aNKT cells | Drug Info | Phase 1 | B-cell small lymphocytic lymphoma | [150] | |
| 140 | CD19/CD22 CAR T cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [151] | |
| 141 | CD19/CD22 CAR T-Cells | Drug Info | Phase 1 | Acute lymphocytic leukaemia | [152] | |
| 142 | CD19/CD22 Chimeric Antigen Receptor T Cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [153] | |
| 143 | CD19CAT-41BBZ CAR T-cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [154] | |
| 144 | CD19t-haNK | Drug Info | Phase 1 | Diffuse large B-cell lymphoma | [155] | |
| 145 | CD20-CD19 cCAR | Drug Info | Phase 1 | B-cell lymphoma | [156] | |
| 146 | Combotox | Drug Info | Phase 1 | leukaemia | [157] | |
| 147 | CTX-101 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [158] | |
| 148 | Donor-derived CD19/22 bispecific CAR-T cells | Drug Info | Phase 1 | leukaemia | [147] | |
| 149 | Duvortuxizumab | Drug Info | Phase 1 | B-cell lymphoma | [14] | |
| 150 | EGFRt/19-28z/4-1BBL CAR T cells | Drug Info | Phase 1 | Chronic lymphocytic leukaemia | [159] | |
| 151 | ET019002 | Drug Info | Phase 1 | B-cell lymphoma | [160] | |
| 152 | ET019003 | Drug Info | Phase 1 | B-cell lymphoma | [161] | |
| 153 | FT-819 | Drug Info | Phase 1 | Chronic lymphocytic leukaemia | [162] | |
| 154 | HuCART19 | Drug Info | Phase 1 | leukaemia | [163] | |
| 155 | Human CD19 targeted T Cells | Drug Info | Phase 1 | Follicular lymphoma | [164] | |
| 156 | IC9-CAR19 cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [165], [166] | |
| 157 | IC9-CAR19 T cells | Drug Info | Phase 1 | B-cell lymphoma | [167] | |
| 158 | ICAR19 CAR-T cells | Drug Info | Phase 1 | B-cell lymphoma | [168] | |
| 159 | IM19 | Drug Info | Phase 1 | Haematological malignancy | [169] | |
| 160 | JWCAR029 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [170] | |
| 161 | KITE-363 | Drug Info | Phase 1 | Diffuse large B-cell lymphoma | [171] | |
| 162 | KUR-502 | Drug Info | Phase 1 | B-cell lymphoma | [172] | |
| 163 | MEDl-551 | Drug Info | Phase 1 | Scleroderma | [173] | |
| 164 | NKX019 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [174] | |
| 165 | Patient-derived CD19- and CD22 specific CAR | Drug Info | Phase 1 | leukaemia | [175] | |
| 166 | PZ01 CAR-T cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [176] | |
| 167 | RG6333 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [177] | |
| 168 | RO7443904 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [178] | |
| 169 | Sleeping Beauty CAR-TCR | Drug Info | Phase 1 | leukaemia | [179] | |
| 170 | TAK-940 | Drug Info | Phase 1 | Haematological malignancy | [180] | |
| 171 | TG-1801 | Drug Info | Phase 1 | B-cell lymphoma | [181] | |
| 172 | TNB-486 | Drug Info | Phase 1 | B-cell lymphoma | [182] | |
| 173 | YTB323 | Drug Info | Phase 1 | Diffuse large B-cell lymphoma | [183] | |
| 174 | Anti-CD19 Chimeric Antigen Receptor T Cells | Drug Info | Clinical trial | leukaemia | [184] | |
| 175 | Anti-CD19/20-CAR vector-transduced T cells | Drug Info | Clinical trial | Acute lymphoblastic leukaemia | [185] | |
| 176 | CAR-CD19 T cell | Drug Info | Clinical trial | B-cell lymphoma | [186] | |
| 177 | CD19-CAR T Cells | Drug Info | Clinical trial | Acute lymphoblastic leukaemia | [187] | |
| 178 | CD19-targeted CAR-T cells | Drug Info | Clinical trial | leukaemia | [188] | |
| 179 | CD19-targeted CAR-T cells | Drug Info | Clinical trial | leukaemia | [189] | |
| 180 | CD19-UCART | Drug Info | Clinical trial | Acute lymphoblastic leukaemia | [190] | |
| 181 | CD19CART | Drug Info | Clinical trial | B-cell lymphoma | [191] | |
| Preclinical Drug(s) | [+] 5 Preclinical Drugs | + | ||||
| 1 | Anti-CD19 CAR T cells | Drug Info | Phase 0 | Hodgkin lymphoma | [192] | |
| 2 | CART19 cells | Drug Info | Phase 0 | Hodgkin lymphoma | [193] | |
| 3 | ATA2431 | Drug Info | Preclinical | B-cell lymphoma | [194] | |
| 4 | ATA3219 | Drug Info | Preclinical | B-cell lymphoma | [194] | |
| 5 | SGN-19A | Drug Info | Preclinical | Haematological malignancy | [195] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| CAR-T-Cell-Therapy | [+] 115 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Axicabtagene ciloleucel | Drug Info | [196] | |||
| 2 | Lisocabtagene maraleucel | Drug Info | [6] | |||
| 3 | CART-19 | Drug Info | [10] | |||
| 4 | CD19 CAR T cells | Drug Info | [11], [199] | |||
| 5 | CAR-T cells targeting CD19 | Drug Info | [15] | |||
| 6 | CART 19 | Drug Info | [16], [17], [201] | |||
| 7 | CART-19 | Drug Info | [18], [202], [203], [204], [205] | |||
| 8 | CART-19 cells | Drug Info | [206], [19] | |||
| 9 | CD19 CAR T cells | Drug Info | [20] | |||
| 10 | CD19-targeting CAR T cells | Drug Info | [21], [22] | |||
| 11 | CTL019 | Drug Info | [23], [24] | |||
| 12 | JCAR015 | Drug Info | [25] | |||
| 13 | KYMRIAH | Drug Info | [26] | |||
| 14 | MB-CART19.1 | Drug Info | [27] | |||
| 15 | XLCART001 | Drug Info | [31] | |||
| 16 | 4SCAR19 cells | Drug Info | [34] | |||
| 17 | Anti-CD19 CAR T cells | Drug Info | [38], [39] | |||
| 18 | Anti-CD19 CAR transduced T cells | Drug Info | [40] | |||
| 19 | Anti-CD19 CAR-T | Drug Info | [41] | |||
| 20 | Anti-CD19 CAR-T cells | Drug Info | [42] | |||
| 21 | Anti-CD19 CAR-T cells | Drug Info | [43] | |||
| 22 | Anti-CD19 CAR-T cells | Drug Info | [44], [45] | |||
| 23 | Anti-CD19 CAR-T cells | Drug Info | [46] | |||
| 24 | Anti-CD19-CAR PBL | Drug Info | [47] | |||
| 25 | Anti-CD19-CAR vector-transduced T cells | Drug Info | [48], [49] | |||
| 26 | Anti-CD19-CAR-T cells | Drug Info | [50] | |||
| 27 | Autologous Anti-CD19CAR-4-1BB-CD3zeta-EGFRt-expressing T Lymphocytes | Drug Info | [55] | |||
| 28 | Autologous CD19-targeting CAR T cells | Drug Info | [56] | |||
| 29 | CAR-T cells targeting CD19 | Drug Info | [57] | |||
| 30 | CARCIK-CD19 | Drug Info | [58] | |||
| 31 | CART-19 cells | Drug Info | [59] | |||
| 32 | CD19 CAR Gene Transduced T Lymphocytes | Drug Info | [64] | |||
| 33 | CD19 CAR T Cells | Drug Info | [65] | |||
| 34 | CD19 CAR T cells | Drug Info | [66] | |||
| 35 | CD19 CAR-T Cells | Drug Info | [67] | |||
| 36 | CD19 CAR-T lymphocytes | Drug Info | [68] | |||
| 37 | CD19 CART | Drug Info | [69] | |||
| 38 | CD19 CART | Drug Info | [70] | |||
| 39 | CD19 specific CAR T cells | Drug Info | [71] | |||
| 40 | CD19 targeted chimeric antigen receptor T cells | Drug Info | [72] | |||
| 41 | CD19-CAR T cell | Drug Info | [73] | |||
| 42 | CD19-directed CAR-T cells | Drug Info | [74] | |||
| 43 | CD19-targeting CAR T Cells | Drug Info | [75] | |||
| 44 | CD19.CAR T Cells | Drug Info | [77] | |||
| 45 | CD19.CAR-T cells | Drug Info | [78] | |||
| 46 | Chimeric Antigen Receptor Modified T cells Targeting CD19 | Drug Info | [79] | |||
| 47 | CLIC-1901 | Drug Info | [80] | |||
| 48 | Humanized CD19 CAR-T cells | Drug Info | [81] | |||
| 49 | IM19 CAR-T | Drug Info | [82], [83] | |||
| 50 | JCAR014 | Drug Info | [214] | |||
| 51 | JCAR017 | Drug Info | [85], [215], [86] | |||
| 52 | PBCAR0191 | Drug Info | [88] | |||
| 53 | PCAR-019 | Drug Info | [89] | |||
| 54 | SCRI-huCAR19v1 | Drug Info | [90] | |||
| 55 | SJCAR19 | Drug Info | [91] | |||
| 56 | TBI-1501 | Drug Info | [93] | |||
| 57 | TriCAR-T-CD19 | Drug Info | [95] | |||
| 58 | UCART019 | Drug Info | [96] | |||
| 59 | 19-28z T CELLS | Drug Info | [97] | |||
| 60 | 4SCAR19 cells | Drug Info | [98] | |||
| 61 | Allogeneic CART-19 | Drug Info | [101] | |||
| 62 | Anti-CD19 CAR-T | Drug Info | [104] | |||
| 63 | Anti-CD19 CAR-T cells | Drug Info | [105] | |||
| 64 | Anti-CD19 CART Cells | Drug Info | [106] | |||
| 65 | Anti-CD19-CAR | Drug Info | [108] | |||
| 66 | Anti-CD19-CAR T | Drug Info | [109] | |||
| 67 | Anti-CD19-CAR T cells | Drug Info | [110] | |||
| 68 | AntiCD19 CART | Drug Info | [112] | |||
| 69 | ARI-0001 cells | Drug Info | [113] | |||
| 70 | C-CAR011 | Drug Info | [116], [117] | |||
| 71 | CAR-T cells targeting CD19 | Drug Info | [119] | |||
| 72 | CAR-T Cells targeting CD19 | Drug Info | [120] | |||
| 73 | CAR19 T cells carrying cytoplasmic activated PD-1 | Drug Info | [121] | |||
| 74 | CART-19 | Drug Info | [122] | |||
| 75 | CART-19 | Drug Info | [123] | |||
| 76 | CART-19 autologous T-cells | Drug Info | [124] | |||
| 77 | CART-19 cells | Drug Info | [125] | |||
| 78 | CART-19 cells | Drug Info | [126] | |||
| 79 | CART-19 T cells | Drug Info | [127] | |||
| 80 | CART19 cell | Drug Info | [129] | |||
| 81 | CD19 CAR T | Drug Info | [134] | |||
| 82 | CD19 CAR T Cells | Drug Info | [135] | |||
| 83 | CD19 CAR T cells | Drug Info | [136], [223] | |||
| 84 | CD19 CAR T-cells | Drug Info | [137] | |||
| 85 | CD19 CAR T-cells | Drug Info | [138] | |||
| 86 | CD19 CAR-T cells | Drug Info | [139] | |||
| 87 | CD19 CAR-T cells | Drug Info | [140] | |||
| 88 | CD19 Redirected Autologous T Cells | Drug Info | [141] | |||
| 89 | CD19+ CAR T Cells | Drug Info | [142] | |||
| 90 | CD19-CAR-T Cells | Drug Info | [144] | |||
| 91 | CD19-CAR-T2 Cells | Drug Info | [145] | |||
| 92 | CD19-directed CAR-T cells | Drug Info | [146] | |||
| 93 | CD19-directed CAR-T cells | Drug Info | [147] | |||
| 94 | CD19-targeted CART cells | Drug Info | [148] | |||
| 95 | CD19-TriCAR-T | Drug Info | [149] | |||
| 96 | CD19.CAR-aNKT cells | Drug Info | [150] | |||
| 97 | CD19CAT-41BBZ CAR T-cells | Drug Info | [154] | |||
| 98 | EGFRt/19-28z/4-1BBL CAR T cells | Drug Info | [159] | |||
| 99 | HuCART19 | Drug Info | [163] | |||
| 100 | Human CD19 targeted T Cells | Drug Info | [164] | |||
| 101 | IC9-CAR19 cells | Drug Info | [165], [166] | |||
| 102 | IC9-CAR19 T cells | Drug Info | [167] | |||
| 103 | ICAR19 CAR-T cells | Drug Info | [168] | |||
| 104 | IM19 | Drug Info | [229], [169] | |||
| 105 | JWCAR029 | Drug Info | [170] | |||
| 106 | PZ01 CAR-T cells | Drug Info | [176] | |||
| 107 | Anti-CD19 Chimeric Antigen Receptor T Cells | Drug Info | [184] | |||
| 108 | CAR-CD19 T cell | Drug Info | [186] | |||
| 109 | CD19-CAR T Cells | Drug Info | [187] | |||
| 110 | CD19-targeted CAR-T cells | Drug Info | [236], [237], [188] | |||
| 111 | CD19-targeted CAR-T cells | Drug Info | [189] | |||
| 112 | CD19-UCART | Drug Info | [190] | |||
| 113 | CD19CART | Drug Info | [191] | |||
| 114 | Anti-CD19 CAR T cells | Drug Info | [192] | |||
| 115 | CART19 cells | Drug Info | [193] | |||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | Blinatumomab | Drug Info | [1] | |||
| 2 | Xmab 5871 | Drug Info | [210], [211] | |||
| 3 | Combotox | Drug Info | [225] | |||
| 4 | Anti-CD22/CD19 mab-toxin conjugate | Drug Info | [238] | |||
| Inhibitor | [+] 6 Inhibitor drugs | + | ||||
| 1 | Inebilizumab | Drug Info | [5] | |||
| 2 | Loncastuximab tesirine | Drug Info | [197] | |||
| 3 | Coltuximab ravtansine | Drug Info | [14] | |||
| 4 | KTE-C19 CAR | Drug Info | [216] | |||
| 5 | AFM11 | Drug Info | [218] | |||
| 6 | AMG 562 | Drug Info | [219] | |||
| Immunostimulant | [+] 1 Immunostimulant drugs | + | ||||
| 1 | Tisagenlecleucel | Drug Info | [2] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 31 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | 4SCAR19 and 4SCAR123 | Drug Info | [33] | |||
| 2 | 4SCAR19 and 4SCAR20 | Drug Info | [33] | |||
| 3 | 4SCAR19 and 4SCAR22 | Drug Info | [33] | |||
| 4 | 4SCAR19 and 4SCAR30 | Drug Info | [33] | |||
| 5 | 4SCAR19 and 4SCAR38 | Drug Info | [33] | |||
| 6 | 4SCAR19 and 4SCAR70 | Drug Info | [33] | |||
| 7 | 4SCAR19/22 T cells | Drug Info | [35] | |||
| 8 | Anti-CD19 and Anti-CD20 CAR-T Cells | Drug Info | [37] | |||
| 9 | Anti-CD19/22-CAR vector-transduced T cells | Drug Info | [51] | |||
| 10 | Anti-CD20 CAR-T cells | Drug Info | [52] | |||
| 11 | AUTO3 | Drug Info | [54], [213] | |||
| 12 | CART-19/22 | Drug Info | [60] | |||
| 13 | CART-19/BCMA | Drug Info | [61] | |||
| 14 | CD19 and CD20 CAR-T Cells | Drug Info | [62] | |||
| 15 | CD19 and CD22 CAR-T Cells | Drug Info | [63] | |||
| 16 | CD19 and CD22 CAR-T Cells | Drug Info | [62] | |||
| 17 | CD19-TCRz-41BB and CD22-TCRz-41BB CAR-T Cells | Drug Info | [76] | |||
| 18 | Anti-CD19 anti-CD20 Bispecific CAR-T | Drug Info | [103] | |||
| 19 | Anti-CD19/BCMA CAR-T cells | Drug Info | [111] | |||
| 20 | BCMA CART and huCART19 | Drug Info | [114] | |||
| 21 | CAR-20/19-T Cells | Drug Info | [118] | |||
| 22 | CART-meso-19 T cells | Drug Info | [128] | |||
| 23 | CART22-65s cells and huCART19 Cells | Drug Info | [130] | |||
| 24 | CARTmeso/19 | Drug Info | [131] | |||
| 25 | CD19-CAR and CD28-CAR T Cells | Drug Info | [143] | |||
| 26 | CD19/CD22 CAR T cells | Drug Info | [151] | |||
| 27 | CD19/CD22 CAR T-Cells | Drug Info | [152] | |||
| 28 | CD19/CD22 Chimeric Antigen Receptor T Cells | Drug Info | [153] | |||
| 29 | Donor-derived CD19/22 bispecific CAR-T cells | Drug Info | [147] | |||
| 30 | Patient-derived CD19- and CD22 specific CAR | Drug Info | [175] | |||
| 31 | Anti-CD19/20-CAR vector-transduced T cells | Drug Info | [185] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| B-lymphocyte surface antigen B4 (CD19) | 100.000 (556/556) | 0.00E+00 | |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
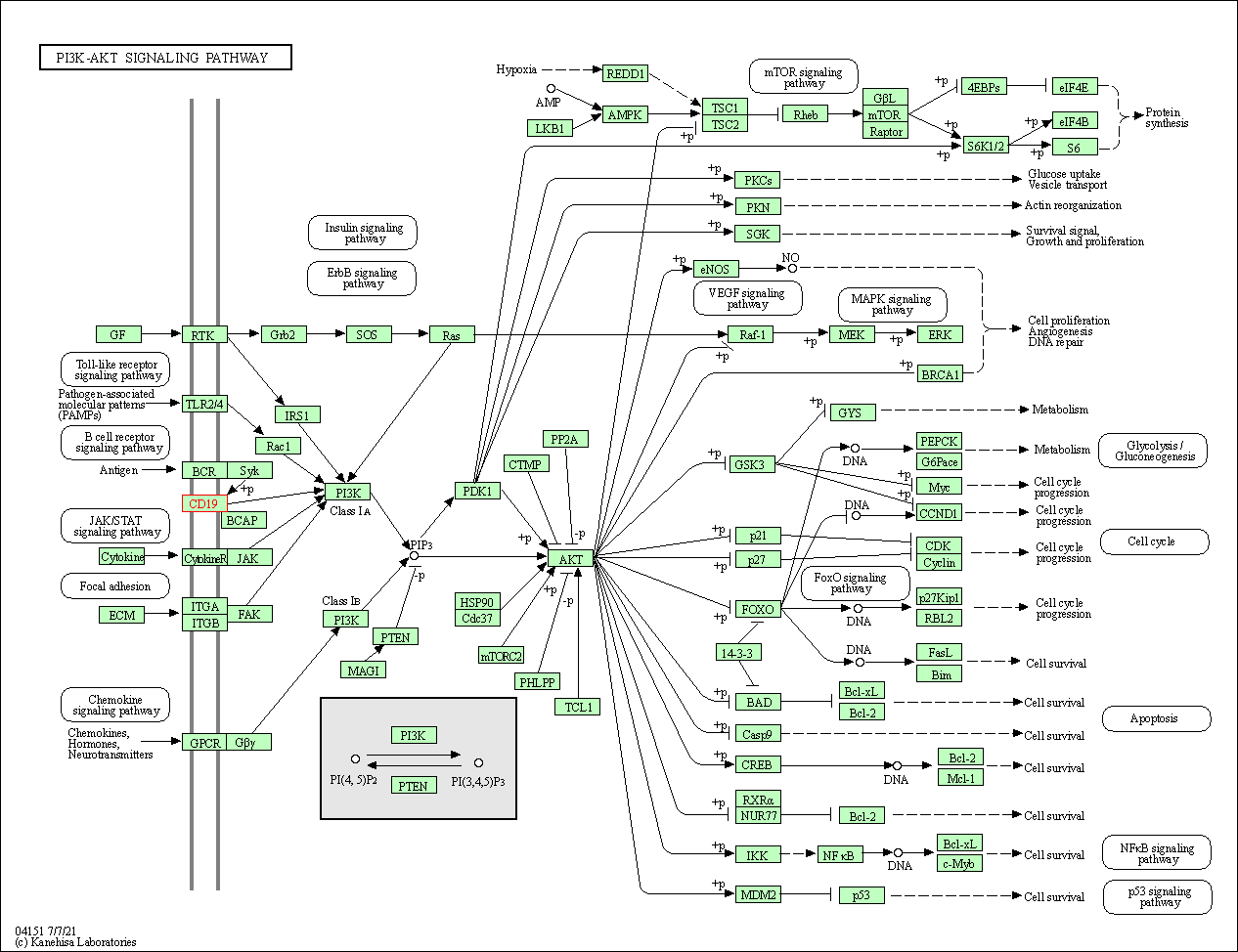
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
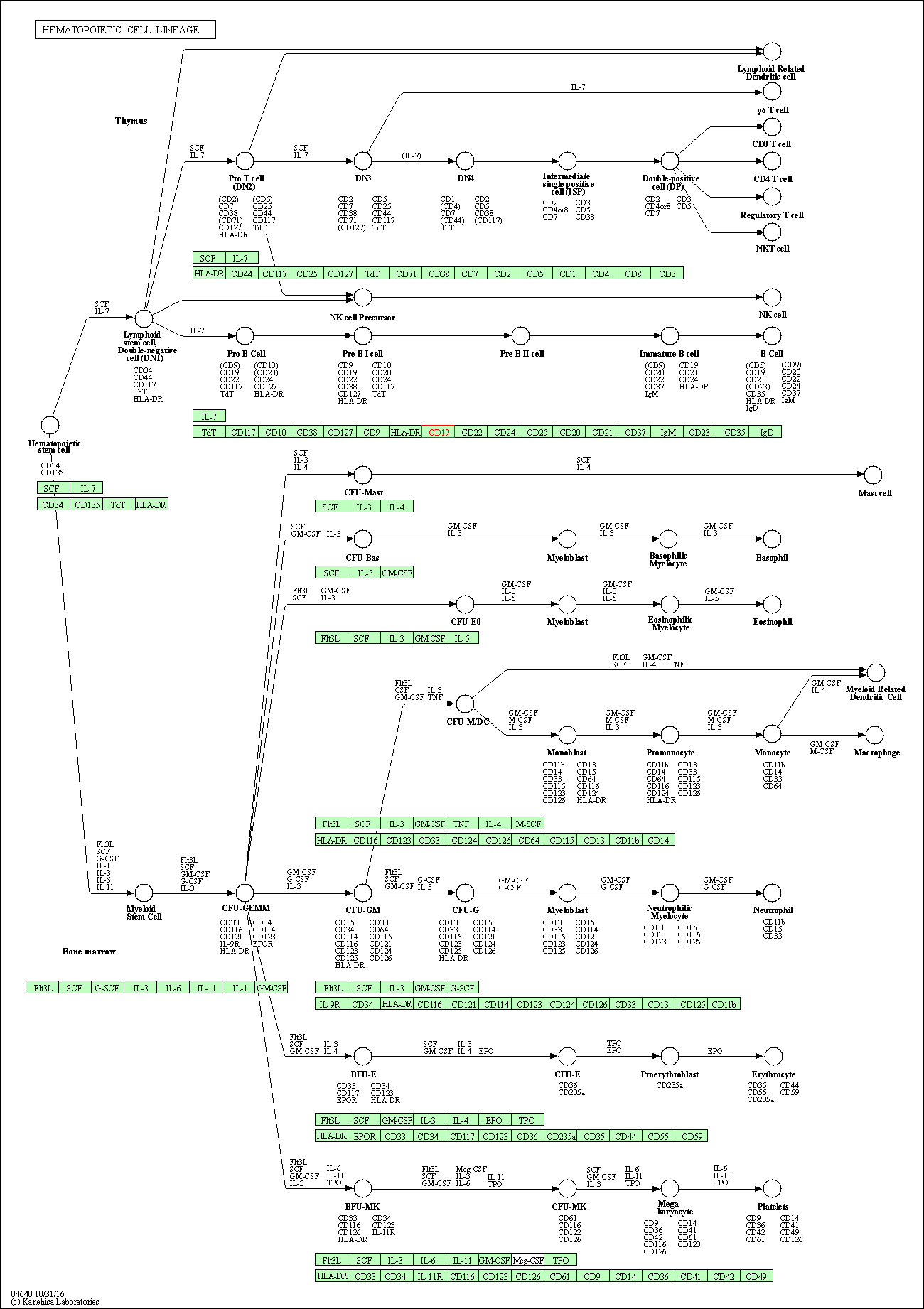
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| B cell receptor signaling pathway | hsa04662 | Affiliated Target |
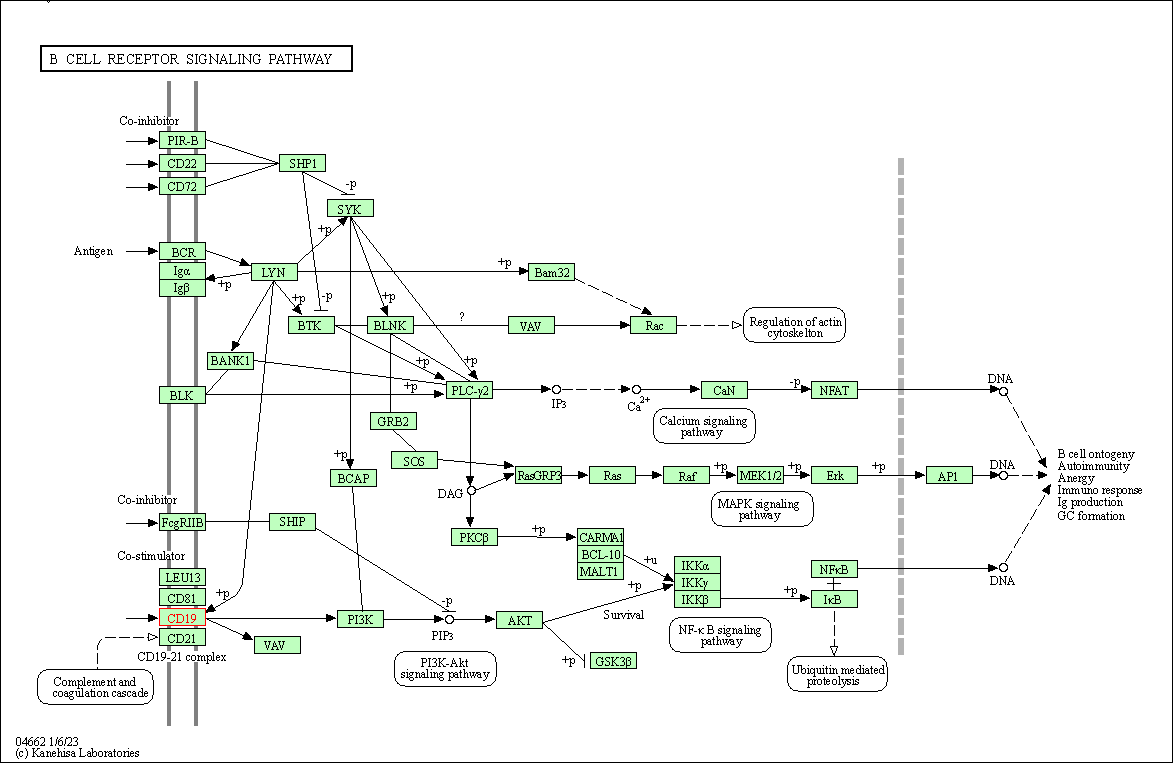
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 14 | Degree centrality | 1.50E-03 | Betweenness centrality | 5.37E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.36E-01 | Radiality | 1.41E+01 | Clustering coefficient | 2.20E-01 |
| Neighborhood connectivity | 4.09E+01 | Topological coefficient | 1.14E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | PI3K-Akt signaling pathway | |||||
| 2 | Hematopoietic cell lineage | |||||
| 3 | B cell receptor signaling pathway | |||||
| 4 | Epstein-Barr virus infection | |||||
| 5 | Primary immunodeficiency | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL-7 Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | B cell activation | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | BCR signaling pathway | |||||
| Reactome | [+] 4 Reactome Pathways | + | ||||
| 1 | PIP3 activates AKT signaling | |||||
| 2 | Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell | |||||
| 3 | Constitutive Signaling by Aberrant PI3K in Cancer | |||||
| 4 | Antigen activates B Cell Receptor (BCR) leading to generation of second messengers | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Human Complement System | |||||
| 2 | Signaling by the B Cell Receptor (BCR) | |||||
| 3 | PIP3 activates AKT signaling | |||||
| 4 | B Cell Receptor Signaling Pathway | |||||
| 5 | Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||||
| REF 2 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 3 | ClinicalTrials.gov (NCT02013167) Blinatumomab Versus Standard of Care Chemotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia (ALL) | |||||
| REF 4 | ClinicalTrials.gov (NCT02393859) Phase 3 Trial of Blinatumomab vs Standard Chemotherapy in Pediatric Subjects With HR First Relapse B-precursor ALL | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. | |||||
| REF 7 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2021 | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. | |||||
| REF 9 | ClinicalTrials.gov (NCT05662241) A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Obexelimab in Patients With IgG4-Related Disease (INDIGO). U.S.National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03027739) CART-19 Cells For MRD Positive CD19+ ALL | |||||
| REF 11 | ClinicalTrials.gov (NCT03391739) CART-19 Cells For R/R B-ALL | |||||
| REF 12 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 13 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 14 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 15 | ClinicalTrials.gov (NCT03196830) CAR-T for R/R B-NHL | |||||
| REF 16 | ClinicalTrials.gov (NCT02935543) CART19 in Adult Patients With Minimal Residual Disease During Upfront Treatment for ALL | |||||
| REF 17 | ClinicalTrials.gov (NCT02906371) Study of the Tocilizumab Optimization Timing for CART19 Associated Cytokine Release Syndrome | |||||
| REF 18 | ClinicalTrials.gov (NCT02030834) Phase IIa Study of Redirected Autologous T Cells Engineered to Contain Anti-CD19 Attached to TCRz and 4-Signaling Domains in Patients With Chemotherapy Relapsed or Refractory CD19+ Lymphomas | |||||
| REF 19 | ClinicalTrials.gov (NCT02794246) CART-19 Post-ASCT for Multiple Myeloma | |||||
| REF 20 | ClinicalTrials.gov (NCT02846584) a Clinical Research of Sequential CAR-T Bridging HSCT in the Treatment of Relapse/Refractory B-cell Malignancies | |||||
| REF 21 | ClinicalTrials.gov (NCT02132624) CD19-targeting 3rd Generation CAR T Cells for Refractory B Cell Malignancy - a Phase I/IIa Trial. | |||||
| REF 22 | ClinicalTrials.gov (NCT03068416) CD19-targeting, 3rd Generation CAR T Cells for Refractory B Cells Malignancy | |||||
| REF 23 | ClinicalTrials.gov (NCT02445248) Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients. U.S. National Institutes of Health. | |||||
| REF 24 | ClinicalTrials.gov (NCT03601442) CTL019 Out of Specification MAP for ALL or DLBCL Patients | |||||
| REF 25 | ClinicalTrials.gov (NCT02535364) Study Evaluating the Efficacy and Safety of JCAR015 in Adult B-cell Acute Lymphoblastic Leukemia (B-ALL). | |||||
| REF 26 | ClinicalTrials.gov (NCT03642626) MT2017-45 :CAR-T Cell Therapy for Heme Malignancies | |||||
| REF 27 | ClinicalTrials.gov (NCT03321123) MB-CART19.1 in Patients With R/R ALL | |||||
| REF 28 | ClinicalTrials.gov (NCT01974479) Pilot Study of Redirected Haploidentical Natural Killer Cell Infusions for B-Lineage Acute Lymphoblastic Leukemia. U.S. National Institutes of Health. | |||||
| REF 29 | ClinicalTrials.gov (NCT05626322) A PHASE 1b/2 STUDY OF PF-07901801, A CD47 BLOCKING AGENT, WITH TAFASITAMAB AND LENALIDOMIDE FOR PARTICIPANTS WITH RELAPSED/REFRACTORY DIFFUSE LARGE B CELL LYMPHOMA NOT ELIGIBLE FOR STEM CELL TRANSPLANTATION. U.S.National Institutes of Health. | |||||
| REF 30 | A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2014 Jan 1;20(1):213-20. | |||||
| REF 31 | ClinicalTrials.gov (NCT03598179) XLCART001 Treatment in Relapsed/Refractory/High-risk B-cell Malignancy Subjects | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035125) | |||||
| REF 33 | ClinicalTrials.gov (NCT03125577) Combination CAR-T Cell Therapy Targeting Hematological Malignancies | |||||
| REF 34 | ClinicalTrials.gov (NCT03050190) A Phase I/II Multiple Center Trial of 4SCAR19 Cells in the Treatment of Relapsed and Refractory B Cell Malignancies | |||||
| REF 35 | ClinicalTrials.gov (NCT03098355) Interleukin-2 Following 4SCAR19/22 T Cells Targeting Refractory and/or Recurrent B Cell Malignancies | |||||
| REF 36 | ClinicalTrials.gov (NCT04416984) A Single-Arm, Open-Label, Phase 1/2 Study Evaluating the Safety, Efficacy, and Cellular Kinetics/Pharmacodynamics of ALLO-501A, an Anti-CD19 Allogeneic CAR T Cell Therapy, and ALLO-647, an Anti-CD52 Monoclonal Antibody, in Subjects With Relapsed/Refractory Large B-Cell Lymphoma (LBCL). U.S.National Institutes of Health. | |||||
| REF 37 | ClinicalTrials.gov (NCT03207178) Sequential Infusion of Anti-CD19 and Anti-CD20 CAR-T Cells Against Relapsed and Refractory B-cell Lymphoma | |||||
| REF 38 | ClinicalTrials.gov (NCT02842138) Autologous CD19 CAR T Cells in Relapsed or Refractory B-cell Lymphoma | |||||
| REF 39 | ClinicalTrials.gov (NCT02247609) Evaluation of 4th Generation Safety-designed CAR T Cells Targeting High-risk and Refractory B Cell Lymphomas | |||||
| REF 40 | ClinicalTrials.gov (NCT03191773) A Study of Anti-CD19 CAR-T Cell Immunotherapy for Refractory /Relapsed B Cell Malignancies | |||||
| REF 41 | ClinicalTrials.gov (NCT02685670) Competitive Transfer of CD19-TCRz-CD28 and CD19-TCRz-CD137 CAR-T Cells for B-cell Leukemia/Lymphoma | |||||
| REF 42 | ClinicalTrials.gov (NCT02851589) Study Evaluating the Efficacy and Safety of PCAR-019 in CD19 Positive Relapsed or Refractory Leukemia and Lymphoma | |||||
| REF 43 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 44 | ClinicalTrials.gov (NCT03366324) Anti-CD19 CAR-T Therapy Combine With HSCT to Treat MRD+ B-cell Malignancies | |||||
| REF 45 | ClinicalTrials.gov (NCT03366350) Anti-CD19 CAR-T Therapy Bridging to HSCT for CD19+ B-Cell Malignancies | |||||
| REF 46 | ClinicalTrials.gov (NCT02782351) Humanized CAR-T Therapy for Treatment of B Cell Malignancy | |||||
| REF 47 | ClinicalTrials.gov (NCT00924326) CAR T Cell Receptor Immunotherapy for Patients With B-cell Lymphoma | |||||
| REF 48 | ClinicalTrials.gov (NCT02081937) CART-19 Immunotherapy in Mantle Cell Lymphoma | |||||
| REF 49 | ClinicalTrials.gov (NCT01864889) Treatment of Relapsed and/or Chemotherapy Refractory B-cell Malignancy by CART19 | |||||
| REF 50 | ClinicalTrials.gov (NCT02672501) A Study to Assess CD19-targeted Immunotherapy T Cells in Patients With Relapsed or Refractory CD19+ B Cell Leukemia | |||||
| REF 51 | ClinicalTrials.gov (NCT03185494) Treatment of Relapsed and/or Chemotherapy Refractory B-cell Malignancy by Tandem CAR T Cells Targeting CD19 and CD22 | |||||
| REF 52 | ClinicalTrials.gov (NCT02737085) the Sequential Therapy of CD19-targeted and CD20-targeted CAR-T Cell Therapy for Diffuse Large B Cell Lymphoma(DLBCL) | |||||
| REF 53 | ClinicalTrials.gov (NCT04404660) A Study of CD19 Targeted CAR T Cell Therapy in Adult Patients With Relapsed or Refractory B Cell Acute Lymphoblastic Leukaemia (ALL). U.S. National Institutes of Health. | |||||
| REF 54 | ClinicalTrials.gov (NCT03289455) CD19 /22 CAR T Cells (AUTO3) for the Treatment of B Cell ALL | |||||
| REF 55 | ClinicalTrials.gov (NCT01865617) Laboratory Treated T Cells in Treating Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Non-Hodgkin Lymphoma, or Acute Lymphoblastic Leukemia | |||||
| REF 56 | ClinicalTrials.gov (NCT02963038) CAR T Cells for Refractory B Cell Malignancy | |||||
| REF 57 | ClinicalTrials.gov (NCT03312205) CAR-T Cells for Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | |||||
| REF 58 | ClinicalTrials.gov (NCT03389035) Transposon-manipulated Allogeneic CARCIK-CD19 Cells in Pediatric and Adult Patients With r/r ALL Post HSCT | |||||
| REF 59 | ClinicalTrials.gov (NCT03544021) CART-19 FOR Relapsed/Refractory Acute Lymphoblastic Leukemia ALL | |||||
| REF 60 | ClinicalTrials.gov (NCT03614858) CD19/CD22-targeted Chimeric Antigen Receptor Engineered T Cell (CART) in B-Cell Acute Lymphoblastic Leukemia. | |||||
| REF 61 | ClinicalTrials.gov (NCT03455972) Study of T Cells Targeting CD19/BCMA (CART-19/BCMA) for High Risk Multiple Myeloma Followed With Auto-HSCT | |||||
| REF 62 | ClinicalTrials.gov (NCT03398967) A Feasibility and Safety Study of Universal Dual Specificity CD19 and CD20 or CD22 CAR-T Cell Immunotherapy for Relapsed or Refractory Leukemia and Lymphoma | |||||
| REF 63 | ClinicalTrials.gov (NCT03468153) Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Relapsed and Refractory Lymphoma | |||||
| REF 64 | ClinicalTrials.gov (NCT02134262) Gene Therapy for B-Cell Non-Hodgkin Lymphoma Using CD19 CAR Gene Transduced T Lymphocytes | |||||
| REF 65 | ClinicalTrials.gov (NCT01475058) CD19 CAR T Cells for B Cell Malignancies After Allogeneic Transplant | |||||
| REF 66 | ClinicalTrials.gov (NCT02772198) T-cells Expressing Anti-CD19 CAR in Pediatric and Young Adults With B-cell Malignancies | |||||
| REF 67 | ClinicalTrials.gov (NCT03263208) CD19 CAR-T Cells for Patients With Relapse and Refractory CD19+ B-ALL. | |||||
| REF 68 | ClinicalTrials.gov (NCT03467256) CD19 T-CAR for Treatment of Children and Young Adults With r/r B-ALL | |||||
| REF 69 | ClinicalTrials.gov (NCT03146533) CD19 CART Cells for Patients With Relapse and Refractory CD19+ B-cell Lymphoma. | |||||
| REF 70 | ClinicalTrials.gov (NCT03232619) CD19-CART Treatment for ALL | |||||
| REF 71 | ClinicalTrials.gov (NCT02028455) A Pediatric and Young Adult Trial of Genetically Modified T Cells Directed Against CD19 for Relapsed/Refractory CD19+ Leukemia | |||||
| REF 72 | ClinicalTrials.gov (NCT03118180) CD19 Targeted Chimeric Antigen Receptor T Cells for B Cell Lymphoma | |||||
| REF 73 | ClinicalTrials.gov (NCT03373071) Anti-CD19 CAR T Cells in Pediatric Patients Affected by Relapsed/Refractory CD19+ ALL and NHL | |||||
| REF 74 | ClinicalTrials.gov (NCT02537977) CD19-directed CAR T Cells Therapy in Relapsed/Refractory B Cell Malignancy | |||||
| REF 75 | ClinicalTrials.gov (NCT02547948) CD19-targeting CAR T Cells for B Cell Lymphoma | |||||
| REF 76 | ClinicalTrials.gov (NCT02903810) Combination Transfer of CD19-TCRz-41BB and CD22-TCRz-41BB CAR-T Cells for B-cell Hematologic Malignancy | |||||
| REF 77 | ClinicalTrials.gov (NCT03676504) Treatment of Patients With Relapsed or Refractory CD19+ Lymphoid Disease With T Cells Expressing a Third-generation CAR | |||||
| REF 78 | ClinicalTrials.gov (NCT02652910) Memory-enriched CAR-T Cells Immunotherapy for B Cell Lymphoma | |||||
| REF 79 | ClinicalTrials.gov (NCT02349698) A Clinical Research of CAR T Cells Targeting CD19 Positive Malignant B-cell Derived Leukemia and Lymphoma | |||||
| REF 80 | ClinicalTrials.gov (NCT03765177) CLIC-1901 for the Treatment of Patients With Relapsed/Refractory CD19 Positive Hematologic Malignancies | |||||
| REF 81 | ClinicalTrials.gov (NCT03275493) Humanized CD19 CAR-T Cells With CRS Suppression Technology for r/r CD19+ Acute Lymphoblastic Leukemia | |||||
| REF 82 | ClinicalTrials.gov (NCT03142646) Safety and Efficacy Evaluation of IM19 CAR-T Cells | |||||
| REF 83 | ClinicalTrials.gov (NCT03173417) Safety and Efficacy Evaluation of IM19 CAR-T Cells (IM19CAR-T) | |||||
| REF 84 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800041075) | |||||
| REF 85 | ClinicalTrials.gov (NCT03436771) Long-term Follow-up Study for Patients Previously Treated With a Juno CAR T-Cell Product | |||||
| REF 86 | ClinicalTrials.gov (NCT03331198) Study Evaluating Safety and Efficacy of JCAR017 in Subjects With Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) | |||||
| REF 87 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800039436) | |||||
| REF 88 | ClinicalTrials.gov (NCT03666000) Dose-escalation Study of Safety of PBCAR0191 in Patients With r/r NHL and r/r B-cell ALL | |||||
| REF 89 | ClinicalTrials.gov (NCT02819583) CAR-T Cell Immunotherapy in CD19 Positive Relapsed or Refractory Leukemia and Lymphoma | |||||
| REF 90 | ClinicalTrials.gov (NCT03684889) CD19-specific CAR T Cells With a Fully Human Binding Domain for CD19+ Leukemia or Lymphoma | |||||
| REF 91 | ClinicalTrials.gov (NCT03573700) Evaluation of CD19-Specific CAR Engineered Autologous T-Cells for Treatment of Relapsed/Refractory CD19+ Acute Lymphoblastic Leukemia | |||||
| REF 92 | Clinical pipeline report, company report or official report of Takeda. | |||||
| REF 93 | ClinicalTrials.gov (NCT03155191) Study of TBI-1501 for Relapsed or Refractory Acute Lymphoblastic Leukemia | |||||
| REF 94 | ClinicalTrials.gov (NCT04323657) TC-110 T Cells in Adults With Relapsed or Refractory Non-Hodgkin Lymphoma or Acute Lymphoblastic Leukemia. U.S. National Institutes of Health. | |||||
| REF 95 | ClinicalTrials.gov (NCT03497533) Treatment of Refractory/Relapsed Non-Hodgkin Lymphoma With TriCAR-T_CD19 | |||||
| REF 96 | ClinicalTrials.gov (NCT03166878) A Study Evaluating UCART019 in Patients With Relapsed or Refractory CD19+ Leukemia and Lymphoma | |||||
| REF 97 | ClinicalTrials.gov (NCT01840566) High Dose Therapy and Autologous Stem Cell Transplantation Followed by Infusion of Chimeric Antigen Receptor (CAR) Modified T-Cells Directed Against CD19+ B-Cells for Relapsed and Refractory Aggressive B Cell Non-Hodgkin Lymphoma | |||||
| REF 98 | ClinicalTrials.gov (NCT02968472) A Phase I Trial of 4SCAR19 Cells in the Treatment of Relapsed and Refractory B Cell Leukemia | |||||
| REF 99 | ClinicalTrials.gov (NCT02106091) Safety Study to Assess AFM11 in Patients With Relapsed and/or Refractory CD19 Positive B-cell NHL or B-precursor ALL. U.S. National Institutes of Health. | |||||
| REF 100 | Clinical pipeline report, company report or official report of Allogene Therapeutics. | |||||
| REF 101 | ClinicalTrials.gov (NCT02799550) Allogeneic CART-19 for Elderly Relapsed/Refractory CD19+ ALL | |||||
| REF 102 | ClinicalTrials.gov (NCT03571828) Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of AMG 562 in Subjects With r/r Diffuse Large B-cell Lymphoma, Mantle Cell Lymphoma, or Follicular Lymphoma. U.S. National Institutes of Health. | |||||
| REF 103 | ClinicalTrials.gov (NCT03271515) Immunotherapy With Bispecific CAR-T Cells for B-Cell Lymphoma, ALL and CLL | |||||
| REF 104 | ClinicalTrials.gov (NCT03110640) Anti-CD19 CAR T Infusion Combined With Allogeneic Stem Cell Transplantation for B-cell Leukemia/Lymphoma | |||||
| REF 105 | ClinicalTrials.gov (NCT03121625) CAR-T Therapy in Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | |||||
| REF 106 | ClinicalTrials.gov (NCT03767725) Anti-BCMA or/and Anti-CD19 CART Cells Treatment of Relapsed Multiple Myeloma | |||||
| REF 107 | ClinicalTrials.gov (NCT00840853) Multi-virus CTLs Expressing CD19 Chimeric Receptors, CD19 Positive Malignancies Post SCT, MULTIPRAT (MULTIPRAT). U.S. National Institutes of Health. | |||||
| REF 108 | ClinicalTrials.gov (NCT01593696) Anti-CD19 White Blood Cells for Children and Young Adults With B Cell Leukemia or Lymphoma | |||||
| REF 109 | ClinicalTrials.gov (NCT02546739) Immunotherapy With CD19 CAR T-cells for B-Cell Lymphoma, ALL and CLL | |||||
| REF 110 | ClinicalTrials.gov (NCT02659943) T Cells Expressing a Fully-human AntiCD19 Chimeric Antigen Receptor for Treating B-cell Malignancies | |||||
| REF 111 | ClinicalTrials.gov (NCT03706547) Anti-CD19/BCMA Bispecific CAR-T Cell Therapy for R/R MM | |||||
| REF 112 | ClinicalTrials.gov (NCT03434769) AntiCD19 Chimeric Antigen Receptor T Cells for Relapsed or Refractory Non Hodgkin Lymphoma | |||||
| REF 113 | ClinicalTrials.gov (NCT03144583) Pilot Study on the Infusion of ARI-0001 Cells in Patients With CD19+ Leukemia or Lymphoma Refractory to Therapy | |||||
| REF 114 | ClinicalTrials.gov (NCT03549442) Up-front CART-BCMA With or Without huCART19 in High-risk Multiple Myeloma | |||||
| REF 115 | ClinicalTrials.gov (NCT04162353) BCMA-CD19 cCAR in Multiple Myeloma and Plasmacytoid Lymphoma. U.S. National Institutes of Health. | |||||
| REF 116 | ClinicalTrials.gov (NCT03299738) A Study Evaluating Safety and Efficacy of C-CAR011 in Subjects With B-NHL | |||||
| REF 117 | ClinicalTrials.gov (NCT03154775) Study of Safety and Efficacy of C-CAR011 in B-NHL Patients | |||||
| REF 118 | ClinicalTrials.gov (NCT03375619) Long-term Follow-up Study of Patients Receiving CAR-20/19-T Cells | |||||
| REF 119 | ClinicalTrials.gov (NCT03086954) Study Evaluating the Efficacy and Safety With CAR-T Immunotherapy for CD19 Positive Lymphoma | |||||
| REF 120 | ClinicalTrials.gov (NCT03186118) Pilot Study of T-APCs Following CAR T Cell Immunotherapy for CD19+ Leukemia | |||||
| REF 121 | ClinicalTrials.gov (NCT03540303) Cytoplasmic Activated PD-1 CAR T Cells in Refractory/Relapsed B Cell Lymphoma | |||||
| REF 122 | ClinicalTrials.gov (NCT03101709) The Safety and Efficacy of CART-19 Cells in Relapse and Refractory Patients With CD19+ B-cell Lymphoma | |||||
| REF 123 | ClinicalTrials.gov (NCT02810223) Efficacy of CART-19 Cell Therapy in B Cell Acute Lymphoblastic Leukemia | |||||
| REF 124 | ClinicalTrials.gov (NCT02476734) FDG-PET/CT Imaging as Early Predictor of DP | |||||
| REF 125 | ClinicalTrials.gov (NCT03291444) CAR-T Cells Combined With Peptide Specific Dendritic Cell in Relapsed/Refractory Leukemia/MDS | |||||
| REF 126 | ClinicalTrials.gov (NCT02924753) The Safety and Efficacy of CART-19 Cells in B-cell Acute Lymphoblastic Leukemia (B-ALL). | |||||
| REF 127 | ClinicalTrials.gov (NCT02135406) CART-19 for Multiple Myeloma | |||||
| REF 128 | ClinicalTrials.gov (NCT02465983) Pilot Study of Autologous T-cells in Patients With Metastatic Pancreatic Cancer | |||||
| REF 129 | ClinicalTrials.gov (NCT03685786) CART19 Cells Treatment of MRD of B Cell Malignancies and Then Auto-HSCT | |||||
| REF 130 | ClinicalTrials.gov (NCT03620058) CART22 Alone or in Combination With huCART19 for ALL | |||||
| REF 131 | ClinicalTrials.gov (NCT03497819) Autologous CARTmeso/19 Against Pancreatic Cancer | |||||
| REF 132 | ClinicalTrials.gov (NCT04156243) CD19 CARvac T Cells for Patients With Relapsed / Refractory B Cell Malignancies. U.S. National Institutes of Health. | |||||
| REF 133 | ClinicalTrials.gov (NCT04231747 ) A Study of CC-97540, CD19-targeted NEX-T Chimeric Antigen Receptor (CAR) T Cells, in Subjects With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma. U.S. National Institutes of Health. | |||||
| REF 134 | ClinicalTrials.gov (NCT03559439) CD19-targeting CAR T Cells in Relapsed or Refractory CD19 Positive B-cell Malignancies | |||||
| REF 135 | ClinicalTrials.gov (NCT01853631) Activated T-Cells Expressing 2nd or 3rd Generation CD19-Specific CAR, Advanced B-Cell NHL, ALL, and CLL (SAGAN) | |||||
| REF 136 | ClinicalTrials.gov (NCT02975687) CD19 CAR T Cells in Patients With Resistant or Refractory CD19+ Acute Lymphoblastic Leukemia | |||||
| REF 137 | ClinicalTrials.gov (NCT03298828) CD19 CAR and PD-1 Knockout Engineered T Cells for CD19 Positive Malignant B-cell Derived Leukemia and Lymphoma | |||||
| REF 138 | ClinicalTrials.gov (NCT02443831) CARPALL: Immunotherapy With CD19 CAR T-cells for CD19+ Haematological Malignancies | |||||
| REF 139 | ClinicalTrials.gov (NCT03671460) CD19 CAR-T Cells for Patients With Relapse and Refractory CD19+ B-ALL. | |||||
| REF 140 | ClinicalTrials.gov (NCT03064269) CAR-T Therapy for Central Nervous System B-cell Acute Lymphocytic Leukemia | |||||
| REF 141 | ClinicalTrials.gov (NCT03030976) A Study of CD19 Redirected Autologous T Cells for CD19 Positive Systemic Lupus Erythematosus (SLE) | |||||
| REF 142 | ClinicalTrials.gov (NCT02529813) CD19+ CAR T Cells for Lymphoid Malignancies | |||||
| REF 143 | ClinicalTrials.gov (NCT02146924) Cellular Immunotherapy in Treating Patients With High-Risk Acute Lymphoblastic Leukemia | |||||
| REF 144 | ClinicalTrials.gov (NCT03574168) CD19-CAR-T Cells in Patients With R/R B-ALL | |||||
| REF 145 | ClinicalTrials.gov (NCT02822326) Phase I Study of CD19-CAR-T2 Cells for Patients With Chemotherapy Resistant or Refractory CD19+ Acute Leukemia | |||||
| REF 146 | ClinicalTrials.gov (NCT03483688) A Phaseb Study Evaluating Safety and Efficacy of C-CAR011 Treatment in B- NHL Subjects | |||||
| REF 147 | ClinicalTrials.gov (NCT03463928) A Feasibility and Safety Study of Concomitant Therapy With Allo-CAR-T Cells and Allo-HSCT in Patients With Relapse or Refractory Leukemia | |||||
| REF 148 | ClinicalTrials.gov (NCT03599375) Immunotherapy With CD19 CART-cells for B Cell Acute Lymphoblastic Leukemia | |||||
| REF 149 | ClinicalTrials.gov (NCT03720496) Treatment of Refractory/Relapsed Non-Hodgkin Lymphoma With CD19-TriCAR-T Cell Therapy | |||||
| REF 150 | ClinicalTrials.gov (NCT03774654) CD19.CAR Allogeneic NKT for Patients With Relapsed or Refractory B-Cell Malignancies (ANCHOR) | |||||
| REF 151 | ClinicalTrials.gov (NCT03241940) CD19/CD22 Chimeric Antigen Receptor T Cells and Chemotherapy in Treating Children or Young Adults With Recurrent or Refractory CD19 Positive B Acute Lymphoblastic Leukemia | |||||
| REF 152 | ClinicalTrials.gov (NCT03448393) CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies | |||||
| REF 153 | ClinicalTrials.gov (NCT03233854) CD19/CD22 Chimeric Antigen Receptor T Cells and Chemotherapy in Treating Patients With Recurrent or Refractory CD19 Positive Diffuse Large B-Cell Lymphoma or B Acute Lymphoblastic Leukemia | |||||
| REF 154 | ClinicalTrials.gov (NCT02935257) Immunotherapy for High Risk/Relapsed CD19+ Acute Lymphoblastic Leukaemia Using CAR T-cells to Target CD19 | |||||
| REF 155 | ClinicalTrials.gov (NCT04052061) QUILT 3.061: CD19 t-haNK in Subjects With Diffuse Large B-Cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 156 | ClinicalTrials.gov (NCT04156178) CD20-CD19 Compound CAR (cCAR) T Cells for Patients With Relapsed /Refractory B Cell Malignancies. U.S. National Institutes of Health. | |||||
| REF 157 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027936) | |||||
| REF 158 | ClinicalTrials.gov (NCT04035434) A Safety and Efficacy Study Evaluating CTX110 in Subjects With Relapsed or Refractory B-Cell Malignancies (CARBON). U.S. National Institutes of Health. | |||||
| REF 159 | ClinicalTrials.gov (NCT03085173) A Trial of "Armored" CAR T Cells Targeting CD19 For Patients With Relapsed CD19+ Hematologic Malignancies | |||||
| REF 160 | ClinicalTrials.gov (NCT03642496) The Clinical Study of Structurally Optimized ET019002-T Cell Therapy for Refractory/Relapsed B-Cell Malignancies. U.S.National Institutes of Health. | |||||
| REF 161 | ClinicalTrials.gov (NCT04014894) Safety and Efficiency Study of ET019003-T Cells in Relapsed/Refractory CD19+ B-Cell Leukemia and Lymphoma. U.S.National Institutes of Health. | |||||
| REF 162 | ClinicalTrials.gov (NCT04629729) FT819 in Subjects With B-cell Malignancies. U.S. National Institutes of Health. | |||||
| REF 163 | ClinicalTrials.gov (NCT02374333) Pilot Study of Redirected Autologous T Cells Engineered to Contain Humanized Anti-CD19 in Patients With Relapsed or Refractory CD19+ Leukemia and Lymphoma Previously Treated With Cell Therapy | |||||
| REF 164 | ClinicalTrials.gov (NCT03720457) Human CD19 Targeted T Cells Injection(CD19 CAR-T) Therapy for Relapsed and Refractory CD19-positive Lymphoma. | |||||
| REF 165 | ClinicalTrials.gov (NCT03016377) Administration of Autologous CAR-T CD19 Antigen With Inducible Safety Switch in Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia | |||||
| REF 166 | ClinicalTrials.gov (NCT03594162) Compassionate Use of CAR T Cells Targeting the CD19 Antigen and Containing the Inducible Caspase 9 Safety Switch | |||||
| REF 167 | ClinicalTrials.gov (NCT03696784) Anti-CD19 CAR-T Cells With Inducible Caspase 9 Safety Switch for B-cell Lymphoma | |||||
| REF 168 | ClinicalTrials.gov (NCT03383952) A Clinical Study of CD19 Targeted CAR-T for Patients With CD19+ Lymphoma and Leukemia | |||||
| REF 169 | ClinicalTrials.gov (NCT03344705) Safety and Efficacy Evaluation of IM19 Cells | |||||
| REF 170 | ClinicalTrials.gov (NCT03344367) Study Evaluating the Safety and Efficacy of JWCAR029 in Adult Subjects With Relapsed and Refractory B-cell Non-Hodgkin Lymphoma | |||||
| REF 171 | ClinicalTrials.gov (NCT04989803) A Phase 1 Open-label, Multicenter Study Evaluating the Safety and Efficacy of KITE-363 or KITE-753, Autologous Anti-CD19/CD20 CAR T-cell Therapies, in Subjects With Relapsed and/or Refractory B-cell Lymphoma. U.S.National Institutes of Health. | |||||
| REF 172 | Clinical pipeline report, company report or official report of Kuur Therapeutics. | |||||
| REF 173 | CD19 as an attractive target for antibody-based therapy. MAbs. 2012 Sep-Oct;4(5):571-7. | |||||
| REF 174 | ClinicalTrials.gov (NCT05020678) A Phase 1 Study of NKX019, a CD19 Chimeric Antigen Receptor Natural Killer (CAR NK) Cell Therapy, in Subjects With B-cell Malignancies. U.S.National Institutes of Health. | |||||
| REF 175 | ClinicalTrials.gov (NCT03330691) A Feasibility and Safety Study of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Leukemia and Lymphoma | |||||
| REF 176 | ClinicalTrials.gov (NCT03281551) Efficacy and Safety of PZ01 Treatment in Patients With r/r CD19+ B-cell Acute Lymphoblastic Leukemia/B Cell Lymphoma | |||||
| REF 177 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 178 | ClinicalTrials.gov (NCT05219513) An Open-Label, Phase 1 Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of RO7443904 in Combination With Glofitamab in Participants With Relapsed/Refractory B-Cell Non-Hodgkin's Lymphoma. U.S.National Institutes of Health. | |||||
| REF 179 | Clinical pipeline report, company report or official report of Ziopharm Oncology. | |||||
| REF 180 | Clinical pipeline report, company report or official report of TAKEDA | |||||
| REF 181 | ClinicalTrials.gov (NCT03804996) Study of TG-1801 in Subjects With B-Cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 182 | ClinicalTrials.gov (NCT04594642) A Study of TNB-486 in Subjects With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. U.S. National Institutes of Health. | |||||
| REF 183 | ClinicalTrials.gov (NCT03960840) CD19-specific CAR-T Cells in CLL/SLL and DLBCL. U.S. National Institutes of Health. | |||||
| REF 184 | ClinicalTrials.gov (NCT03624686) Production of Clinical-grade Anti-CD19 Chimeric Antigen Receptor T Cells for Refractory B-cell Malignancies | |||||
| REF 185 | ClinicalTrials.gov (NCT03097770) Treatment of Relapsed and/or Chemotherapy Refractory B-cell Malignancy by Tandem CAR T Cells Targeting CD19 and CD20 | |||||
| REF 186 | ClinicalTrials.gov (NCT03302403) Clinical Study of Redirected Autologous T Cells With a Chimeric Antigen Receptor in Patients With Malignant Tumors | |||||
| REF 187 | ClinicalTrials.gov (NCT03423706) Clinical Studies of New Model Haploidentical Hematopoietic Stem Cell Transplantation | |||||
| REF 188 | ClinicalTrials.gov (NCT02735291) Study Evaluating the Efficacy and Safety With CAR-T for Recurrent or Refractory Acute Non T Lymphocyte Leukemia | |||||
| REF 189 | ClinicalTrials.gov (NCT03564977) CD19-targeted CAR-T Cell Therapy for MRD+ B-cell Malignancies After Autologous Stem Cell Transplantation | |||||
| REF 190 | ClinicalTrials.gov (NCT03229876) Safety and Efficacy Evaluation of CD19-UCART | |||||
| REF 191 | ClinicalTrials.gov (NCT02813837) Chimeric Antigen Receptor T Cells (CART) Therapy in Refractory/Relapsed B Cell Hematologic Malignancies | |||||
| REF 192 | ClinicalTrials.gov (NCT02277522) CD19 Redirected Autologous T Cells for Hodgkin Lymphoma | |||||
| REF 193 | ClinicalTrials.gov (NCT02624258) Pilot Study of Non-Viral, RNA-Redirected Autologous T Cells in Patients With Refractory or Relapsed Hodgkin Lymphoma | |||||
| REF 194 | Clinical pipeline report, company report or official report of Atara Biotherapeutics. | |||||
| REF 195 | 2011 Pipeline of Seattle Genetics. | |||||
| REF 196 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services | |||||
| REF 197 | Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021 May 13;137(19):2634-2645. | |||||
| REF 198 | Emerging B-Cell Therapies in Systemic Lupus Erythematosus. Ther Clin Risk Manag. 2021 Jan 14;17:39-54. | |||||
| REF 199 | ClinicalTrials.gov (NCT03391726) CART-19 Cells for R/R B-cell Lymphoma | |||||
| REF 200 | A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014 Dec 4;124(24):3553-60. | |||||
| REF 201 | ClinicalTrials.gov (NCT02640209) Pilot Trial Of Autologous T Cells Engineered To Express Anti-CD19 Chimeric Antigen Receptor (CART19)In Combination With Ibrutinib In Patients With Relapsed Or Refractory CD19+ Chronic Lymphocytic Leukemia (CLL)Or Small Lymphocytic Lymphoma (SLL) | |||||
| REF 202 | ClinicalTrials.gov (NCT01029366) CART19 to Treat B-Cell Leukemia or Lymphoma That Are Resistant or Refractory to Chemotherapy | |||||
| REF 203 | ClinicalTrials.gov (NCT01747486) CD19 Redirected Autologous T Cells | |||||
| REF 204 | ClinicalTrials.gov (NCT01626495) Phase I/IIA Study of CART19 Cells for Patients With Chemotherapy Resistant or Refractory CD19+ Leukemia and Lymphoma | |||||
| REF 205 | ClinicalTrials.gov (NCT02030847) Study of Redirected Autologous T Cells Engineered to Contain Anti-CD19 Attached to TCR and 4-1BB Signaling Domains in Patients With Chemotherapy Resistant or Refractory Acute Lymphoblastic Leukemia | |||||
| REF 206 | ClinicalTrials.gov (NCT01551043) Allo CART-19 Protocol | |||||
| REF 207 | A nonfucosylated human antibody to CD19 with potent B-cell depletive activity for therapy of B-cell malignancies. Cancer Immunol Immunother. 2010 Feb;59(2):257-65. | |||||
| REF 208 | Clinical pipeline report, company report or official report of MedImmune (2011). | |||||
| REF 209 | Tafasitamab: First Approval. Drugs. 2020 Nov;80(16):1731-1737. | |||||
| REF 210 | Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor complex and Fc receptor IIb inhibitory receptor.Arthritis Rheumatol.2014 May;66(5):1153-64. | |||||
| REF 211 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 212 | Clinical pipeline report, company report or official report of Autolus Therapeutics. | |||||
| REF 213 | ClinicalTrials.gov (NCT03287817) CD19/22 CAR T Cells (AUTO3) for the Treatment of Diffuse Large B Cell Lymphoma | |||||
| REF 214 | ClinicalTrials.gov (NCT02706405) JCAR014 and Durvalumab in Treating Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma | |||||
| REF 215 | ClinicalTrials.gov (NCT02631044) Study Evaluating the Safety and Pharmacokinetics of JCAR017 in B-cell Non-Hodgkin Lymphoma (TRANSCEND-NHL-001) | |||||
| REF 216 | Clinical pipeline report, company report or official report of Kite Pharma. | |||||
| REF 217 | Clinical pipeline report, company report or official report of TCR2 Therapeutics. | |||||
| REF 218 | A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs. 2015;7(3):584-604. | |||||
| REF 219 | Clinical pipeline report, company report or official report of Amgen. | |||||
| REF 220 | Cytotoxic T cells transduced with chimeric anti-CD19 receptors prevent engraftment of primary lymphoblastic leukemia in vivo. Leukemia. 2010 May;24(5):1080-4. | |||||
| REF 221 | Clinical pipeline report, company report or official report of iCell Gene Therapeutics. | |||||
| REF 222 | A Study of CC-97540, CD19-targeted NEX-T Chimeric Antigen Receptor (CAR) T Cells, in Subjects With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma | |||||
| REF 223 | ClinicalTrials.gov (NCT03029338) CD19 CAR T Cells in Patients With Relapsed or Refractory CD19 Positive B-cell Lymphoma | |||||
| REF 224 | Clinical pipeline report, company report or official report of iCell Gene Therapeutics. | |||||
| REF 225 | A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia.Br J Haematol.2011 Aug;154(4):471-6. | |||||
| REF 226 | Off-the-shelf' allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020 Mar;19(3):185-199. | |||||
| REF 227 | Novel CD19-specific gamma-delta TCR-T cells in relapsed or refractory diffuse large B-cell lymphoma. J Hematol Oncol. 2023 Jan 21;16(1):5. | |||||
| REF 228 | Clinical pipeline report, company report or official report of Fate Therapeutics. | |||||
| REF 229 | ClinicalTrials.gov (NCT03528421) Assessment of Safety and Efficacy of IM19 for Relapsed or Refractory NHL Patients | |||||
| REF 230 | Clinical pipeline report, company report or official report of Gilead | |||||
| REF 231 | Clinical pipeline report, company report or official report of Nkarta | |||||
| REF 232 | Clinical pipeline report, company report or official report of Genentch | |||||
| REF 233 | Clinical pipeline report, company report or official report of TG Theraputics. | |||||
| REF 234 | Clinical pipeline report, company report or official report of Teneobio. | |||||
| REF 235 | Clinical pipeline report, company report or official report of Novartis. | |||||
| REF 236 | ClinicalTrials.gov (NCT02728882) Study Evaluating the Efficacy and Safety With CAR-T for Recurrent or Refractory Diffuse Large B Cell Lymphoma | |||||
| REF 237 | ClinicalTrials.gov (NCT03488160) CAR-T Treatment for Relapse / Refractory Type Safety and Effectiveness of Lymphoma | |||||
| REF 238 | Immunotoxins against CD19 and CD22 are effective in killing precursor-B acute lymphoblastic leukemia cells in vitro. Leukemia. 2000 May;14(5):853-8. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

