Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T65197
(Former ID: TTDS00144)
|
|||||
| Target Name |
Erythropoietin Receptor (EPOR)
|
|||||
| Synonyms |
Full-length form; EPO-R
Click to Show/Hide
|
|||||
| Gene Name |
EPOR
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Anemia [ICD-11: 3A00-3A9Z] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 3 | Transplant rejection [ICD-11: NE84] | |||||
| Function |
Mediates erythropoietin-induced erythroblast proliferation and differentiation. Upon EPO stimulation, EPOR dimerizes triggering the JAK2/STAT5 signaling cascade. In some cell types, can also activate STAT1 and STAT3. May also activate the LYN tyrosine kinase. Receptor for erythropoietin.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MDHLGASLWPQVGSLCLLLAGAAWAPPPNLPDPKFESKAALLAARGPEELLCFTERLEDL
VCFWEEAASAGVGPGNYSFSYQLEDEPWKLCRLHQAPTARGAVRFWCSLPTADTSSFVPL ELRVTAASGAPRYHRVIHINEVVLLDAPVGLVARLADESGHVVLRWLPPPETPMTSHIRY EVDVSAGNGAGSVQRVEILEGRTECVLSNLRGRTRYTFAVRARMAEPSFGGFWSAWSEPV SLLTPSDLDPLILTLSLILVVILVLLTVLALLSHRRALKQKIWPGIPSPESEFEGLFTTH KGNFQLWLYQNDGCLWWSPCTPFTEDPPASLEVLSERCWGTMQAVEPGTDDEGPLLEPVG SEHAQDTYLVLDKWLLPRNPPSEDLPGPGGSVDIVAMDEGSEASSCSSALASKPSPEGAS AASFEYTILDPSSQLLRPWTLCPELPPTPPHLKYLYLVVSDSGISTDYSSGDSQGAQGGL SDGPYSNPYENSLIPAAEPLPPSYVACS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T89LO8 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Darbepoetin alfa | Drug Info | Approved | Anemia | [2] | |
| 2 | Epoetin alfa | Drug Info | Approved | Anemia | [3] | |
| 3 | Methoxy polyethylene glycol-epoetin beta | Drug Info | Approved | Kidney transplant rejection | [4], [5] | |
| 4 | RHuEPO | Drug Info | Approved | Solid tumour/cancer | [5], [6] | |
| Clinical Trial Drug(s) | [+] 4 Clinical Trial Drugs | + | ||||
| 1 | Epoetin zeta | Drug Info | Phase 3 | Anemia | [7] | |
| 2 | ErepoXen | Drug Info | Phase 3 | Anemia | [8] | |
| 3 | Hematide | Drug Info | Phase 3 | Anemia | [9] | |
| 4 | Cibinetide | Drug Info | Phase 2 | Depression | [10] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | GlycoPEGylated erythropoietin | Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | [11] | |
| 2 | Albupoietin | Drug Info | Terminated | Anemia | [12] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Agonist | [+] 5 Agonist drugs | + | ||||
| 1 | Darbepoetin alfa | Drug Info | [13] | |||
| 2 | Epoetin alfa | Drug Info | [1], [14] | |||
| 3 | ErepoXen | Drug Info | [17] | |||
| 4 | Hematide | Drug Info | [18], [19], [20] | |||
| 5 | Cibinetide | Drug Info | [21] | |||
| Activator | [+] 1 Activator drugs | + | ||||
| 1 | Methoxy polyethylene glycol-epoetin beta | Drug Info | [4] | |||
| Stimulator | [+] 7 Stimulator drugs | + | ||||
| 1 | RHuEPO | Drug Info | [15] | |||
| 2 | BBT-009 | Drug Info | [15] | |||
| 3 | BBT-021 | Drug Info | [15] | |||
| 4 | Erythropoietin | Drug Info | [15] | |||
| 5 | P-1116 | Drug Info | [15] | |||
| 6 | PEG-EPO | Drug Info | [15] | |||
| 7 | PT-401 | Drug Info | [15] | |||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | Epoetin zeta | Drug Info | [16] | |||
| 2 | GlycoPEGylated erythropoietin | Drug Info | [22] | |||
| 3 | Albupoietin | Drug Info | [23] | |||
| 4 | EPO peptide mimetics | Drug Info | [15] | |||
| 5 | EPO-derived peptide | Drug Info | [15] | |||
| 6 | Nova-EPO | Drug Info | [15] | |||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | 3,5 DIBROMOTYROSINE | Drug Info | [24] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
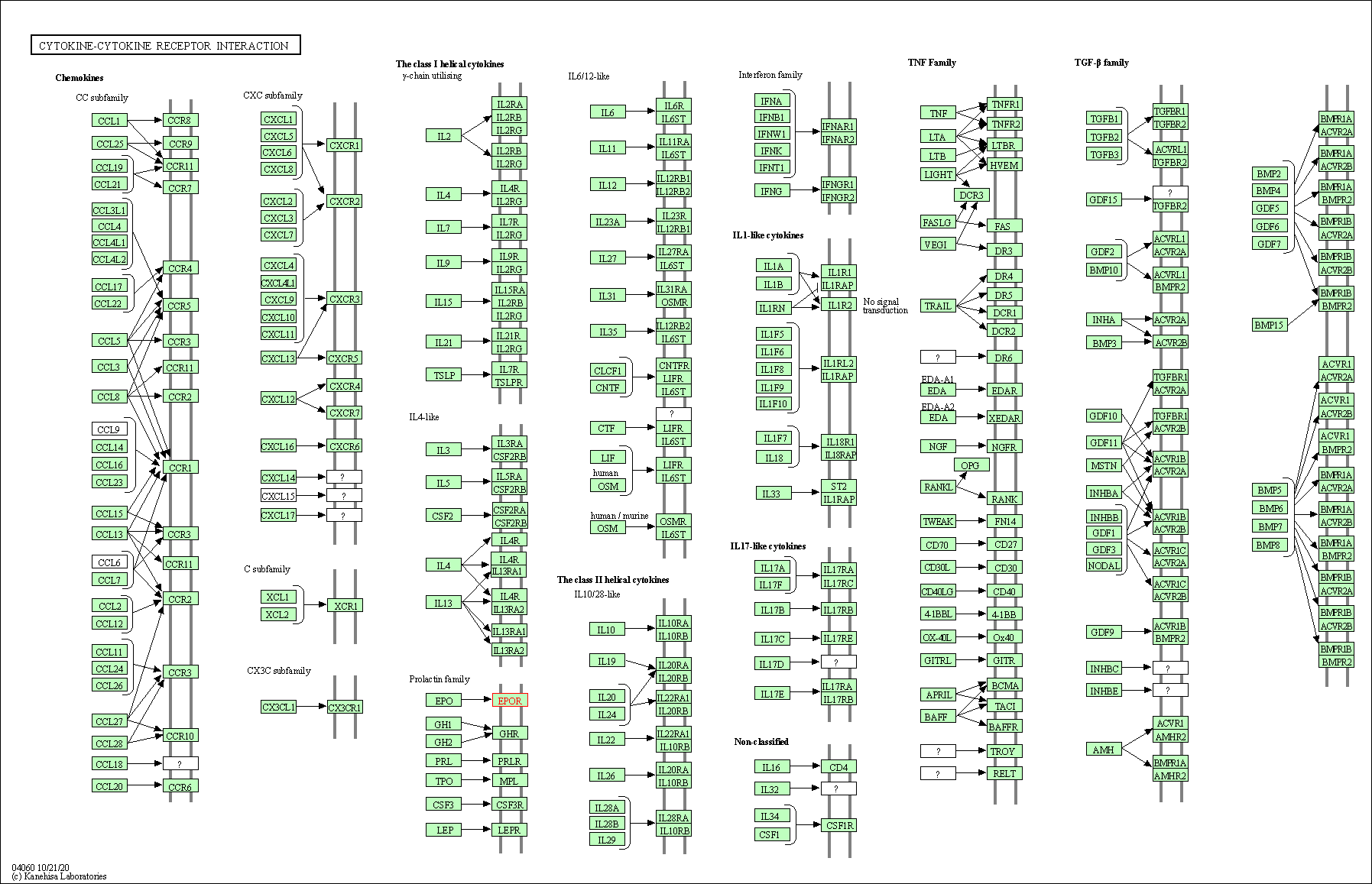
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
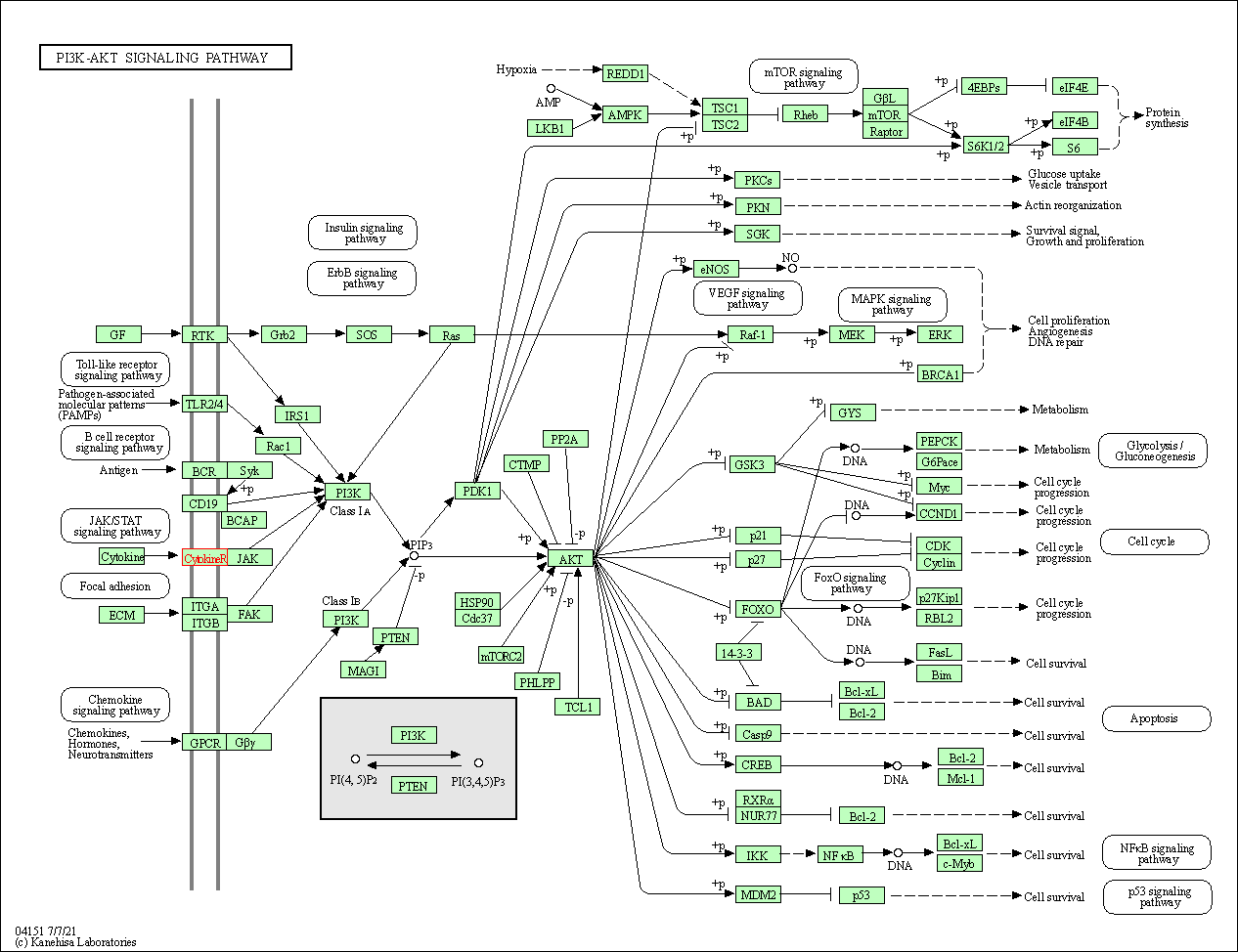
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
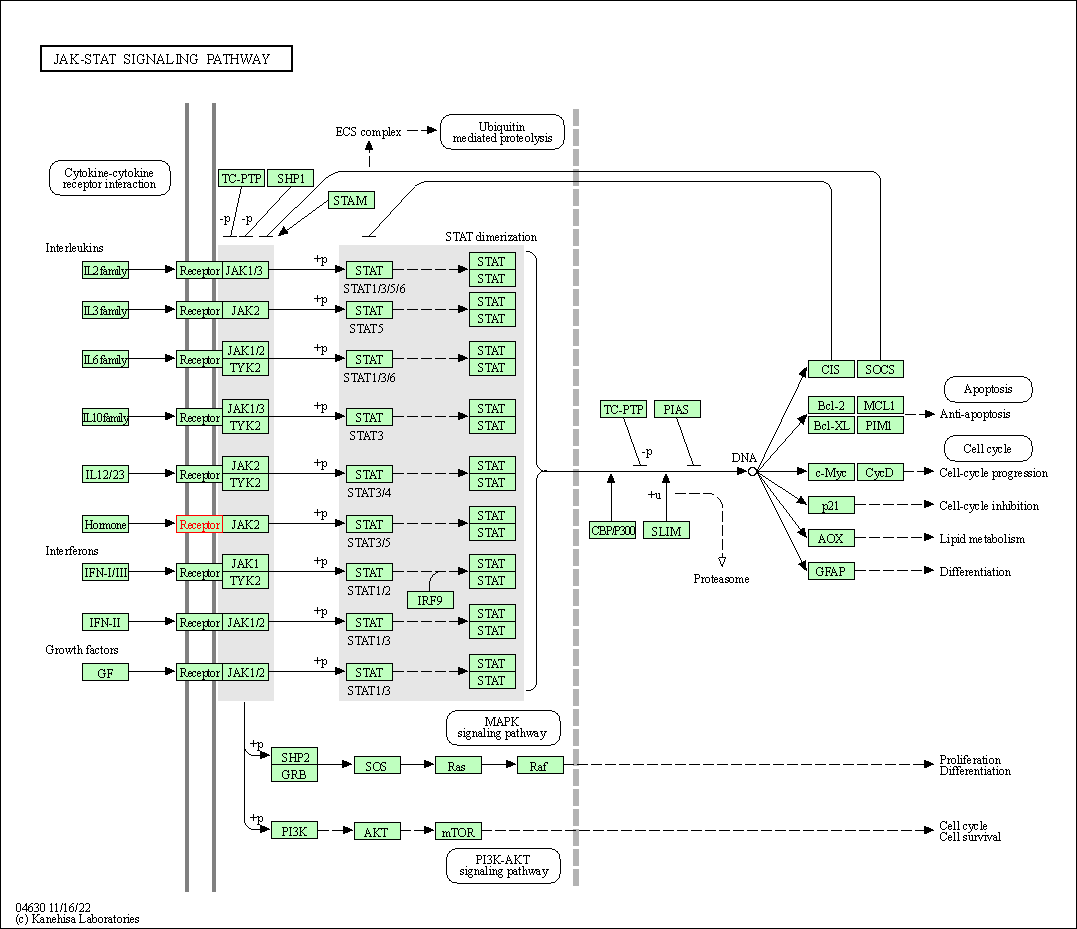
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
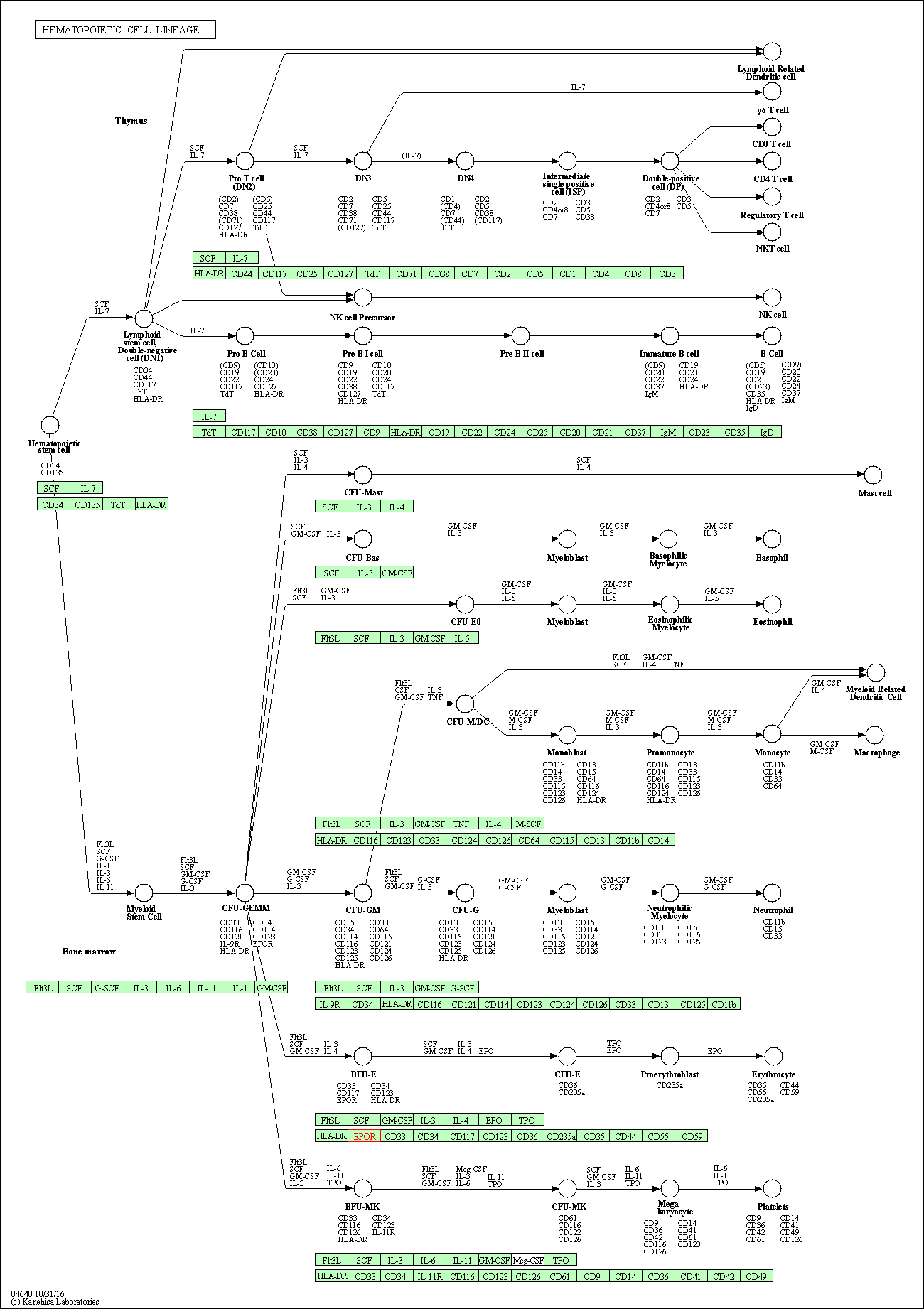
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 19 | Degree centrality | 2.04E-03 | Betweenness centrality | 4.48E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.31E-01 | Radiality | 1.41E+01 | Clustering coefficient | 3.22E-01 |
| Neighborhood connectivity | 4.26E+01 | Topological coefficient | 1.30E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | PI3K-Akt signaling pathway | |||||
| 3 | Jak-STAT signaling pathway | |||||
| 4 | Hematopoietic cell lineage | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TGF_beta_Receptor Signaling Pathway | |||||
| PID Pathway | [+] 2 PID Pathways | + | ||||
| 1 | Signaling events mediated by Stem cell factor receptor (c-Kit) | |||||
| 2 | EPO signaling pathway | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | EPO Receptor Signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2007 Standards, Options, and Recommendations: use of erythropoiesis-stimulating agents (ESA: epoetin alfa, epoetin beta, and darbepoetin) for the management of anemia in children with cancer. PediatrBlood Cancer. 2009 Jul;53(1):7-12. | |||||
| REF 2 | Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009 Mar;14(1):67-84. | |||||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (BLA) 103951. | |||||
| REF 4 | 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9. | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 6 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008056) | |||||
| REF 7 | Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008 Mar;24(3):625-37. | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033828) | |||||
| REF 9 | Clinical pipeline report, company report or official report of Takeda. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035038) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018390) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017518) | |||||
| REF 13 | A cytosolic domain of the erythropoietin receptor contributes to endoplasmic reticulum-associated degradation. Eur J Biochem. 1999 Jul;263(2):410-9. | |||||
| REF 14 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1718). | |||||
| REF 16 | Long-term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Adv Ther. 2008 Nov;25(11):1215-28. | |||||
| REF 17 | Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica. 2006 Aug;91(8):1076-83. | |||||
| REF 18 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | |||||
| REF 19 | Peginesatide and erythropoietin stimulate similar erythropoietin receptor-mediated signal transduction and gene induction events. Exp Hematol. 2012 Jul;40(7):575-87. | |||||
| REF 20 | Anemia in chronic kidney disease: status of new therapies. Curr Opin Nephrol Hypertens. 2009 Mar;18(2):112-5. | |||||
| REF 21 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 22 | Design of Homogeneous, MonoPEGylated Erythropoietin Analogs With Preserved In Vitro Bioactivity. Exp Hematol. 2006 June; 34(6): 697-704. | |||||
| REF 23 | Clinical pipeline report, company report or official report of cogenesys. | |||||
| REF 24 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

