Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00HBR
|
||||
| Former ID |
DIB015545
|
||||
| Drug Name |
AIKO-150
|
||||
| Synonyms |
Opioid neutral antagonists (iv, pain), Aiko Biotechnology; 6-beta-naltrexol
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Opiate dependence [ICD9: 304; ICD10:F11] | Phase 1 | [522558] | ||
| Company |
Aiko Biotechnology Inc
|
||||
| Structure |
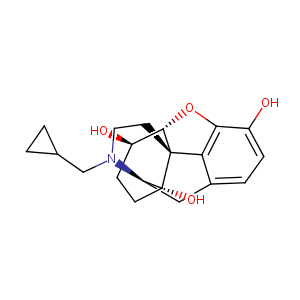
|
Download2D MOL |
|||
| Formula |
C20H25NO4
|
||||
| Canonical SMILES |
c1cc(c2c3c1C[C@@H]1[C@]4([C@@]3([C@H]([C@@H](CC4)O)O2)C<br />CN1CC1CC1)O)O
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Mu-type opioid receptor | Target Info | Antagonist | [551690] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| Pathway Interaction Database | IL4-mediated signaling events | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

