Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00WJE
|
||||
| Former ID |
DIB009040
|
||||
| Drug Name |
SUN-1334H
|
||||
| Synonyms |
Histamine H1 receptor antagonist (oral, allergy) Sun Pharmaceutical
|
||||
| Company |
Sun Pharmaceutical Industries Ltd
|
||||
| Structure |
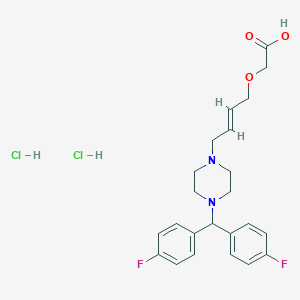
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Histamine H1 receptor | Target Info | Antagonist | [529341] | |
| PANTHER Pathway | Histamine H1 receptor mediated signaling pathway | ||||
| References | |||||
| Ref 529341 | Preclinical efficacy and safety pharmacology of SUN-1334H, a potent orally active antihistamine agent. Drugs R D. 2008;9(2):93-112. | ||||
| Ref 548589 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026836) | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

