Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00YLW
|
||||
| Former ID |
DAP000368
|
||||
| Drug Name |
Dolasetron
|
||||
| Synonyms |
Dolasetronum; Dolasteron; Anzemet (TN); Dolasetron (INN); Dolasetron [INN:BAN]; Dolasetronum [INN-Latin]; 3-oxooctahydro-2h-2,6-methanoquinolizin-8-yl 1h-indole-3-carboxylate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Nausea; Vomiting [ICD9: 787, 787.0; ICD10:R11] | Approved | [536361] | ||
| Therapeutic Class |
Antiemetics
|
||||
| Company |
Aventis Pharmaceuticals Inc.
|
||||
| Structure |
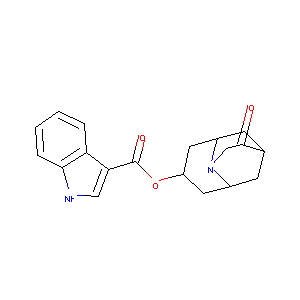
|
Download2D MOL |
|||
| Formula |
C19H20N2O3
|
||||
| Canonical SMILES |
C1C2CC3CC(CC1N3CC2=O)OC(=O)C4=CNC5=CC=CC=C54
|
||||
| InChI |
1S/C19H20N2O3/c22-18-10-21-12-5-11(18)6-13(21)8-14(7-12)24-19(23)16-9-20-17-4-2-1-3-15(16)17/h1-4,9,11-14,20H,5-8,10H2/t11?,12-,13+,14?
|
||||
| InChIKey |
UKTAZPQNNNJVKR-AKJUYKBHSA-N
|
||||
| CAS Number |
CAS 115956-12-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
A04AA04
|
||||
| SuperDrug CAS ID |
cas=115956122
|
||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine receptor 3A | Target Info | Antagonist | [537011] | |
| KEGG Pathway | Serotonergic synapse | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| PANTHER Pathway | 5HT3 type receptor mediated signaling pathway | ||||
| Reactome | Ligand-gated ion channel transport | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Iron uptake and transport | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

