Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00ZUU
|
||||
| Former ID |
DIB011022
|
||||
| Drug Name |
ONO-5334
|
||||
| Synonyms |
Bone resorption inhibitor (tablet/oral, osteoporosis/osteopenia), Ono; Cathepsin K inhibitor (tablet/oral formulation, osteoporosis/osteopenia), Ono
|
||||
| Indication | Osteopenia [ICD9: 733.9; ICD10:M85.8] | Discontinued in Phase 2 | [522118] | ||
| Company |
Ono Pharmaceutical Co Ltd
|
||||
| Structure |
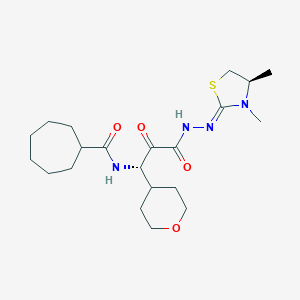
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Cathepsin K | Target Info | Inhibitor | [531317], [532506] | |
| WikiPathways | RANKL/RANK Signaling Pathway | ||||
| Osteoclast Signaling | |||||
| References | |||||
| Ref 531317 | New approach for osteoporosis treatment: cathepsin K inhibitor, ONO-5334. Clin Calcium. 2011 Jan;21(1):64-9. | ||||
| Ref 532506 | Population pharmacokinetic and pharmacodynamic modeling of different formulations of ONO-5334, cathepsin K inhibitor, in Caucasian and Japanese postmenopausal females. J Clin Pharmacol. 2014 Jan;54(1):23-34. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

