Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01FSE
|
||||
| Former ID |
DNC011829
|
||||
| Drug Name |
U-69593
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [539024] | ||
| Structure |
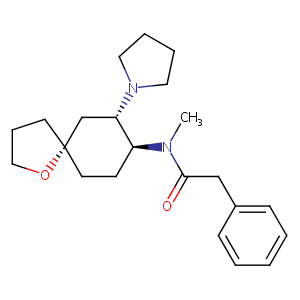
|
Download2D MOL |
|||
| Formula |
C22H32N2O2
|
||||
| InChI |
InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1
|
||||
| InChIKey |
PGZRDDYTKFZSFR-ONTIZHBOSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
841864, 7980860, 10233764, 26756545, 44434631, 48032743, 49689203, 50066063, 50093889, 53787917, 57338019, 76304920, 103177815, 103841744, 104373646, 117567761, 124892412, 128914721, 134349034, 135062519, 135651213, 135651293, 136946583, 142250937, 144205579, 144239943, 162224354, 163426066, 170466866, 172914645, 198966748, 227014322, 252451182
|
||||
| Target and Pathway | |||||
| Target(s) | Mu-type opioid receptor | Target Info | Inhibitor | [528255] | |
| Kappa-type opioid receptor | Target Info | Inhibitor | [530889] | ||
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||||
| Enkephalin releaseP00026:Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||||
| Opioid prodynorphin pathway | |||||
| Pathway Interaction Database | IL4-mediated signaling events | ||||
| References | |||||
| Ref 528255 | Bioorg Med Chem Lett. 2006 Aug 15;16(16):4291-5. Epub 2006 Jun 13.Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. | ||||
| Ref 530889 | J Med Chem. 2010 May 27;53(10):4212-22.Conformationally constrained kappa receptor agonists: stereoselective synthesis and pharmacological evaluation of 6,8-diazabicyclo[3.2.2]nonane derivatives. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

