Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01ZUV
|
||||
| Former ID |
DNCL002334
|
||||
| Drug Name |
GDC-0425
|
||||
| Indication | Lymphoma [ICD9: 202.8, 208.9; ICD10:C81-C86] | Phase 1 | [523494] | ||
| Company |
Genentech
|
||||
| Structure |
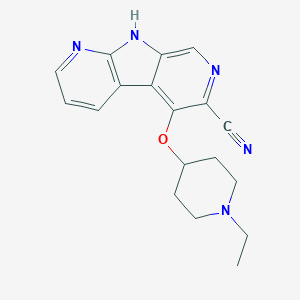
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Serine/threonine-protein kinase Chk1 | Target Info | Modulator | [549773] | |
| Pathway Interaction Database | Fanconi anemia pathway | ||||
| p73 transcription factor network | |||||
| ATR signaling pathway | |||||
| Circadian rhythm pathway | |||||
| p53 pathway | |||||
| Reactome | Activation of ATR in response to replication stress | ||||
| Processing of DNA double-strand break ends | |||||
| Presynaptic phase of homologous DNA pairing and strand exchange | |||||
| G2/M DNA damage checkpoint | |||||
| Ubiquitin Mediated Degradation of Phosphorylated Cdc25A | |||||
| Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

