Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02EZM
|
||||
| Former ID |
DNAP001689
|
||||
| Drug Name |
Fospropofol disodium
|
||||
| Synonyms |
Lusedra (TN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Eisai
|
||||
| Structure |
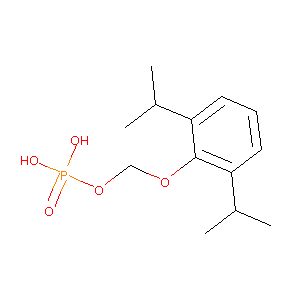
|
Download2D MOL |
|||
| Formula |
C13H19Na2O5P
|
||||
| Canonical SMILES |
CC(C)C1=C(C(=CC=C1)C(C)C)OCOP(=O)([O-])[O-].[Na+].[Na+]
|
||||
| InChI |
1S/C13H21O5P.2Na/c1-9(2)11-6-5-7-12(10(3)4)13(11)17-8-18-19(14,15)16;;/h5-7,9-10H,8H2,1-4H3,(H2,14,15,16);;/q;2*+1/p-2
|
||||
| InChIKey |
LWYLQNWMSGFCOZ-UHFFFAOYSA-L
|
||||
| CAS Number |
CAS 258516-87-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
N01AX10
|
||||
| Target and Pathway | |||||
| Target(s) | Glutamate receptor AMPA subtype | Target Info | Modulator | [551871] | |
| References | |||||
| Ref 526010 | General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. JPharmacol Exp Ther. 2001 Apr;297(1):338-51. | ||||
| Ref 529941 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

