Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02VFB
|
||||
| Former ID |
DIB016678
|
||||
| Drug Name |
Miproxifene
|
||||
| Synonyms |
Miproxifene phosphate; TAT-59
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Discontinued in Phase 3 | [544738] | ||
| Company |
Taiho Pharmaceutical Co Ltd
|
||||
| Structure |
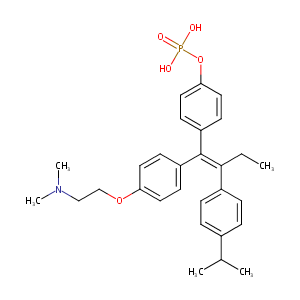
|
Download2D MOL |
|||
| Formula |
C29H35NO2
|
||||
| Canonical SMILES |
P(=O)(Oc1ccc(/C(=C(/c2ccc(cc2)C(C)C)\CC)/c2ccc(cc2)OCCN<br />(C)C)cc1)(O)O
|
||||
| CAS Number |
CAS 115767-74-3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Estrogen receptor | Target Info | Modulator | [525689] | |
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | ||||
| Signaling events mediated by HDAC Class II | |||||
| Plasma membrane estrogen receptor signaling | |||||
| LKB1 signaling events | |||||
| Regulation of Telomerase | |||||
| ATF-2 transcription factor network | |||||
| AP-1 transcription factor network | |||||
| FOXM1 transcription factor network | |||||
| Validated nuclear estrogen receptor alpha network | |||||
| Signaling mediated by p38-alpha and p38-beta | |||||
| FOXA1 transcription factor network | |||||
| References | |||||
| Ref 525689 | Estrogen agonistic/antagonistic effects of miproxifene phosphate (TAT-59)Shibata J1, Toko T, Saito H, Lykkesfeldt AE, Fujioka A, Sato K, Hashimoto A, Wierzba K, Yamada Y.Author information1Hanno Research Center, Taiho Pharmaceutical Co., Ltd., 1-27 Misugidai, Hanno-City, Saitama, 357-8527, Japan.Erratum inCancer Chemother Pharmacol 2000;46(2):172. AbstractPURPOSE: We evaluated miproxifene phosphate (TAT-59) to elucidate its efficacy in antiestrogen therapy for breast cancer patients and to assess its tissue-selective estrogenic/antiestrogenic activity.METHODS: Using DP-TAT-59, a major and active metabolite of TAT-59, an in vitro cell growth inhibition test was performed. Antitumor activity was determined using TAT-59 against human tumor xenografts of the MCF-7 and the Br-10 cell lines andMCF-7-derived tamoxifen-resistant cell lines, R-27 and FST-1. The antitumor activity of DP-TAT-59 and DM-DP-TAT-59, major metabolites of TAT-59 found in human blood following a TAT-59 dose, was also examined after intravenous administration to experimental animals. The residual estrogenic activity of TAT-59, evaluated in terms of bone and lipid metabolism in ovariectomized rats, was then comparedwith that of tamoxifen.RESULTS: DP-TAT-59 significantly inhibited the proliferation of estrogen receptor-positive MCF-7 and T-47D tumor cells in the presence of 1 nM estradiol. TAT-59, given to mice bearing MCF-7 or Br-10 xenografts, at the dose level of 5 mg/kg, exerted a significant growth inhibitory effect that was stronger than that of tamoxifen. Moreover, R-27 and FST-1 tumors, which show a resistance to tamoxifen, responded strongly to TAT-59, suggesting that TAT-59 might be effective against tumors resistant to tamoxifen. The metabolites of TAT-59, DP-TAT-59 and DM-DP-TAT-59, showed similar antitumor activity. Both TAT-59 and tamoxifen suppressed the decrease in bone density and reduced the blood cholesterol levels in ovariectomized rats, suggesting that the estrogenic activity of TAT-59 is comparable to that of tamoxifen.CONCLUSIONS: On the basis of the above results, one may expect TAT-59 to become an effective drug in patients with tumors less sensitive to tamoxifen, while its estrogenic activity as determined by bone and lipid metabolism is similar to that of tamoxifen.PMID: 10663628 [PubMed - indexed for MEDLINE] ShareMeSH Terms, SubstancesMeSH TermsAnimalsAntineoplastic Agents, Hormonal/pharmacologyBreast Neoplasms/pathology*Cell Division/drug effectsDrug Screening Assays, AntitumorEstradiol/pharmacologyEstrogen Antagonists/pharmacology*FemaleHumansLipid MetabolismMiceMice, Inbred BALB CRatsRats, Sprague-DawleyReceptors, Estrogen/physiologyTamoxifen/analogs & derivatives*Tamoxifen/pharmacologyTransplantation, HeterologousTumor Cells, Cultured/drug effectsSubstancesAntineoplastic Agents, HormonalEstrogen AntagonistsReceptors, EstrogenTamoxifenTAT 59EstradiolLinkOut - more resourcesOther Literature SourcesAccess more work from the authors - ResearchGateMedicalBreast Cancer - MedlinePlus Health InformationMiscellaneousESTRADIOL - Hazardous Substances Data BankTAMOXIFEN - Hazardous Substances Data BankPubMed Commons home Cancer Chemother Pharmacol. 2000;45(2):133-41. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

