Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03RRJ
|
||||
| Former ID |
DNC000803
|
||||
| Drug Name |
Iodine-131-tositumomab
|
||||
| Drug Type |
Antibody
|
||||
| Indication | Discovery agent | Phase 2 | [523216] | ||
| Structure |
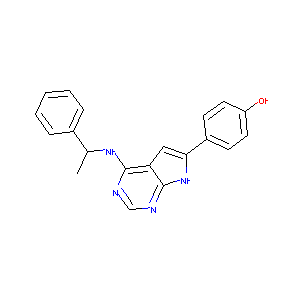
|
Download2D MOL |
|||
| Formula |
C44H39ClN8O5
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | B-lymphocyte antigen CD20 | Target Info | [535062], [536721] | ||
| KEGG Pathway | Hematopoietic cell lineage | ||||
| References | |||||
| Ref 535062 | A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000 Nov 1;96(9):2934-42. | ||||
| Ref 536721 | Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008 Aug 1;112(3):830-5. Epub 2008 May 23. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

