Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04DMQ
|
||||
| Former ID |
DIB010695
|
||||
| Drug Name |
Fabesetron
|
||||
| Synonyms |
FK-1052
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Irritable bowel syndrome [ICD9: 564.1, 787.91; ICD10:A09, K58, K59.1] | Discontinued in Phase 2 | [544894] | ||
| Company |
Fujisawa Pharmaceutical Co Ltd
|
||||
| Structure |
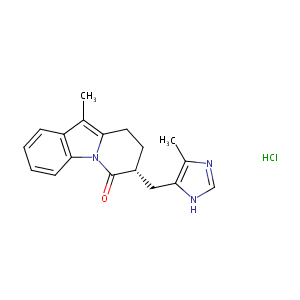
|
Download2D MOL |
|||
| Formula |
C18H19N3O
|
||||
| Canonical SMILES |
n12c(c(c3c2cccc3)C)CC[C@@H](C1=O)Cc1c(nc[nH]1)C.Cl
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 4 receptor | Target Info | Modulator | ||
| 5-HT 3 receptor | Target Info | Modulator | |||
| PathWhiz Pathway | Excitatory Neural Signalling Through 5-HTR 4 and Serotonin | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

