Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04KAM
|
||||
| Former ID |
DNC011463
|
||||
| Drug Name |
Acetic acid 2-hexylsulfanyl-phenyl ester
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [534753] | ||
| Structure |
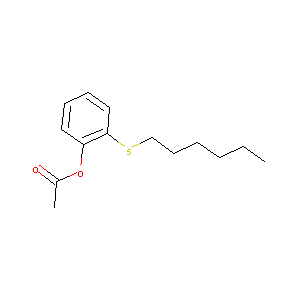
|
Download2D MOL |
|||
| Formula |
C14H20O2S
|
||||
| Canonical SMILES |
CCCCCCSC1=CC=CC=C1OC(=O)C
|
||||
| InChI |
1S/C14H20O2S/c1-3-4-5-8-11-17-14-10-7-6-9-13(14)16-12(2)15/h6-7,9-10H,3-5,8,11H2,1-2H3
|
||||
| InChIKey |
IIONGZARVQOSAZ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin G/H synthase 1 | Target Info | Inhibitor | [534753] | |
| BioCyc Pathway | C20 prostanoid biosynthesis | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

