Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04MBS
|
||||
| Former ID |
DIB005355
|
||||
| Drug Name |
BMS-182657
|
||||
| Indication | Cardiovascular disorder [ICD10:I00-I99] | Terminated | [533583] | ||
| Company |
Bristol-Myers Squibb Co
|
||||
| Structure |
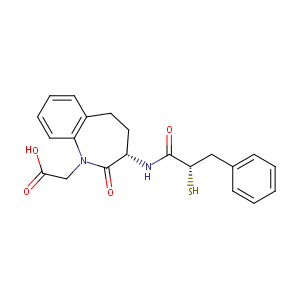
|
Download2D MOL |
|||
| Canonical SMILES |
N1(C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)S)CCc2c1cccc2)CC(=<br />O)O
|
||||
| CAS Number |
CAS 160135-56-8
|
||||
| Target and Pathway | |||||
| Target(s) | Neutral endopeptidase | Target Info | Modulator | [533583] | |
| Angiotensin-converting enzyme | Target Info | Modulator | [533583] | ||
| NetPath Pathway | EGFR1 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| PathWhiz Pathway | Angiotensin Metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

