Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05RHI
|
||||
| Former ID |
DAP000760
|
||||
| Drug Name |
Trifluridine
|
||||
| Synonyms |
Fluridine; TFDU; TRIFLUOROTHYMIDINE; Trifluoridine; Trifluoromethyldeoxyuridine; Trifluridina; Trifluridinum; Virophta; Viroptic; Trifluorothymine deoxyriboside; CF3dUrd; F3DThd; F3T; F3TDR; HS-0007; Trifluridina [INN-Spanish]; Trifluridine [USAN:INN]; Trifluridinum [INN-Latin]; Viroptic (TN); Trifluridine (USP/INN);Viroptic, Trifluorothymidine, Trifluridine; Alpha,alpha,alpha-Trifluorothymidine; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl)pyrimidine-2,4-dione; 2'-Deoxy-5-(trifluoromethyl)uridine; 2'-Deoxy-5-trifluoromethyluridine; 5-(Trifluoromethyl)-2'-deoxyuridine; 5-(Trifluoromethyl)deoxyuridine; 5-Trifluoro-2'-deoxythymidine; 5-Trifluoromethyl-2'-deoxyuridine; 5-Trifluoromethyl-2-deoxyuridine; 5-Trifluorothymidine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiviral Agents
|
||||
| Company |
Monarch Pharmaceuticals
|
||||
| Structure |
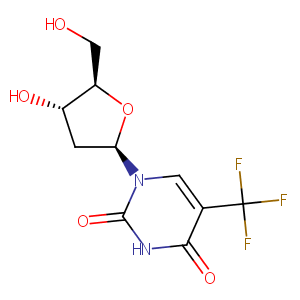
|
Download2D MOL |
|||
| Formula |
C10H11F3N2O5
|
||||
| InChI |
InChI=1S/C10H11F3N2O5/c11-10(12,13)4-2-15(9(19)14-8(4)18)7-1-5(17)6(3-16)20-7/h2,5-7,16-17H,1,3H2,(H,14,18,19)/t5-,6+,7+/m0/s1
|
||||
| InChIKey |
VSQQQLOSPVPRAZ-RRKCRQDMSA-N
|
||||
| CAS Number |
CAS 70-00-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
626314, 7847457, 7980831, 8139891, 8153936, 12014784, 12149237, 14751529, 14849367, 24900027, 29225252, 46506192, 50054714, 56422883, 57323294, 77174799, 87560183, 93815015, 103321204, 104023317, 104253375, 104311541, 118048237, 124757493, 124800643, 125164297, 126667216, 129521334, 131404691, 134223275, 134338045, 134971818, 135681084, 135692274, 136369804, 137000771, 137005241, 137213977, 144205964, 152058901, 160963778, 163835390, 170464911, 172092201, 172919625, 174483143, 175266936, 175612130, 176484794, 179116934
|
||||
| SuperDrug ATC ID |
S01AD02
|
||||
| SuperDrug CAS ID |
cas=000070008
|
||||
| Target and Pathway | |||||
| Target(s) | Thymidylate synthase | Target Info | Inhibitor | [536764] | |
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| Ref 538271 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074311. | ||||
| Ref 543253 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8697). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

