| Drug General Information |
| Drug ID |
D06CWR
|
| Former ID |
DNC008625
|
| Drug Name |
4-(6-Methoxynaphthalen-2-yl)isoquinoline
|
| Drug Type |
Small molecular drug
|
| Structure |
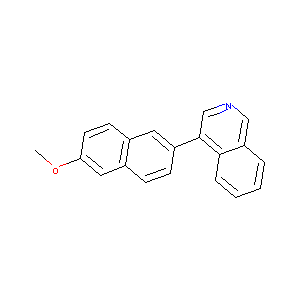
|
Download
2D MOL
3D MOL
|
| Formula |
C20H15NO
|
| Canonical SMILES |
COC1=CC2=C(C=C1)C=C(C=C2)C3=CN=CC4=CC=CC=C43
|
| InChI |
1S/C20H15NO/c1-22-18-9-8-14-10-16(7-6-15(14)11-18)20-13-21-12-17-4-2-3-5-19(17)20/h2-13H,1H3
|
| InChIKey |
SGBOBLSELUHWRG-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| References |
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.