| Drug General Information |
| Drug ID |
D06JHV
|
| Former ID |
DIB013320
|
| Drug Name |
Flumatinib
|
| Synonyms |
HH-GV-678
|
| Drug Type |
Small molecular drug
|
| Company |
Shanghai Institute of Materia Medica of the Chinese Academy of Sciences
|
| Structure |
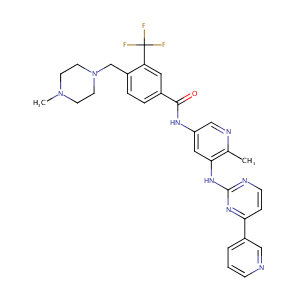
|
Download
2D MOL
3D MOL
|
| Formula |
C29H29F3N8O
|
| Canonical SMILES |
FC(F)(c1cc(C(=O)Nc2cnc(c(c2)Nc2nc(ccn2)c2cnccc2)C)ccc1C<br />N1CCN(CC1)C)F
|
| PubChem Compound ID |
|
| Target and Pathway |
| References |
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.