Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06LFV
|
||||
| Former ID |
DCL000179
|
||||
| Drug Name |
NM-702
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Angina pectoris [ICD9: 413; ICD10:I20] | Phase 3 | [544180] | ||
| Company |
Nissan Chemical America
|
||||
| Structure |
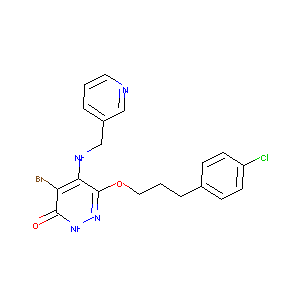
|
Download2D MOL |
|||
| Formula |
C19H19BrCl2N4O2
|
||||
| Canonical SMILES |
C1=CC(=CN=C1)CNC2=C(C(=O)NN=C2OCCCC3=CC=C(C=C3)Cl)Br.Cl
|
||||
| InChIKey |
QWGUGDYWUADMGB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 878796-94-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Phosphodiesterase | Target Info | Inhibitor | [536325] | |
| Thromboxane-A synthase | Target Info | Inhibitor | [536325] | ||
| BioCyc Pathway | C20 prostanoid biosynthesis | ||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

