Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06YFA
|
||||
| Former ID |
DAP000282
|
||||
| Drug Name |
Bromocriptine
|
||||
| Synonyms |
Bagren; Bromergocryptine; Bromocriptin; Bromocriptina; Bromocriptinum; Bromocryptin; Bromocryptine; Bromoergocriptine; Bromoergocryptine; Ergoset; Parlodel; Bromocriptine [BAN]; Bromocriptine methanesulfonate; Parlodel Snaptabs; Alti-Bromocriptine; Apo-Bromocriptine; Bromocriptina [INN-Spanish]; Bromocriptinum [INN-Latin]; CB-154; Parlodel (TN); Bromocriptine (USAN/INN); Bromocriptine [USAN:BAN:INN]; Ergocryptine, 2-bromo-(8CI); (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxoergotaman; (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)ergotaman-3',6',18-trione; (5'alpha)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-2'-(propan-2-yl)-3',6',18-trioxoergotaman; (5'alpha)-2-bromo-12'-hydroxy-5'-isobutyl-2'-isopropyl-3',6',18-trioxoergotaman; (6aR,9R)-5-Bromo-N-((2R,5S,10aS,10bS)-10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxooctahydro-2H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide; 2-Bromo-12'-hydroxy-2'-(1-methylethyl)-5'-alpha-(2-methylpropyl)ergotamin-3',6',18-trione; 2-Bromo-alpha-ergocryptine; 2-Bromo-alpha-ergokryptin; 2-Bromo-alpha-ergokryptine; 2-Bromoergocryptine Methanesulfonate; 2-Bromoergokryptine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiparkinson Agents
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
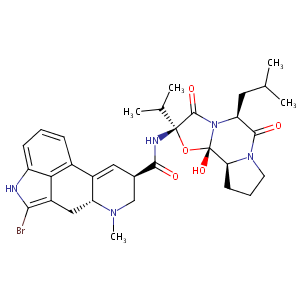
|
Download2D MOL |
|||
| Formula |
C32H40BrN5O5
|
||||
| InChI |
InChI=1S/C32H40BrN5O5/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39)/t18-,23-,24+,25+,31-,32+/m1/s1
|
||||
| InChIKey |
OZVBMTJYIDMWIL-AYFBDAFISA-N
|
||||
| CAS Number |
CAS 25614-03-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9074, 605475, 7978821, 8171939, 11405760, 11466149, 11467269, 11485837, 14765834, 14765835, 17397319, 24262982, 34673282, 46505504, 47217056, 47440541, 47589227, 47589228, 47885652, 48415647, 49658626, 49965589, 50872033, 53789332, 57311062, 85788755, 90340796, 92712670, 93166622, 103170197, 103936294, 104309997, 117390441, 124749509, 124886913, 126625420, 126656565, 127298886, 127298887, 127298888, 127298889, 127298890, 127298891, 127298892, 127298893, 127298894, 127298895, 127298896, 127298897, 127298898
|
||||
| ChEBI ID |
ChEBI:3181
|
||||
| SuperDrug ATC ID |
G02CB01; N04BC01
|
||||
| SuperDrug CAS ID |
cas=025614033
|
||||
| Target and Pathway | |||||
| Target(s) | D(2) dopamine receptor | Target Info | Agonist | [537057], [537165], [537694] | |
| References | |||||
| Ref 536285 | Novel pharmacological targets for the treatment of Parkinson's disease. Nat Rev Drug Discov. 2006 Oct;5(10):845-54. | ||||
| Ref 540438 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 35). | ||||
| Ref 537057 | Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009 Feb 4;29(5):1538-43. | ||||
| Ref 537165 | Silymarin BIO-C, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine. 2009 Sep;16(9):839-44. Epub 2009 Mar 20. | ||||
| Ref 537694 | The behavioral toxicity of bromocriptine in patients with psychiatric illness. J Clin Psychopharmacol. 1989 Dec;9(6):417-22. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

