Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08OKJ
|
||||
| Former ID |
DAP001312
|
||||
| Drug Name |
Iron Dextran
|
||||
| Synonyms |
Chinofer; Dexferrum; Dextrofer; Eisendextran; Fenate; Ferridextran; Ferrodextran; Ferroglucin; Hematran; Icar; Imfergen; Imferon; Imperon; Imposil; Infed; Iron; Norferan; Polyfer; Proferdex; Prolongal; Ursoferran; Dextran iron complex; Eisendextran [German]; Fero Gradumet; Ferric dextran; Iron dextran complex; Iron dextran injection; Iron hydrogenated dextran; Ironorm injection; Sulfuric acid; A 100; B 75; Dextrofer 75; Ferdex 100; Ferroglukin 75; Myofer 100; A 100 (Pharmaceutical); American Regent Brand of Iron-Dextran Complex; Dextran-Iron Complex; Fe-Dextran; GlaxoSmithKline Brand of Iron-Dextran Complex; Goldline Brand of Iron-Dextran Complex; Hauck Brand of Iron-Dextran Complex; Hawthron Brand of Iron-Dextran Complex; Iro-Jex; Iron-dextran complex; Sanofi Brand of Iron-Dextran Complex; Sulfuric acid, iron salt; Sulphuric acid, iron salt; Vortech Brand of Iron-Dextran Complex; Watson Brand of Iron-Dextran Complex; Sulfuric acid iron salt (1:1); Sulfuric acid, iron salt (1:); Sulfuric acid iron(2+) salt (1:1)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Iron Supplement
|
||||
| Structure |
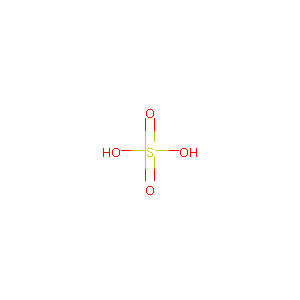
|
Download2D MOL |
|||
| Formula |
FeH2O4S
|
||||
| Canonical SMILES |
OS(=O)(=O)O.[Fe]
|
||||
| InChI |
1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)
|
||||
| InChIKey |
MVZXTUSAYBWAAM-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 9004-66-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
B05AA05
|
||||
| Target and Pathway | |||||
| Target(s) | Hemoglobin | Target Info | Activator | [536828] | |
| KEGG Pathway | African trypanosomiasis | ||||
| Malaria | |||||
| WikiPathways | Binding and Uptake of Ligands by Scavenger Receptors | ||||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | |||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | |||||
| Factors involved in megakaryocyte development and platelet production | |||||
| Folate Metabolism | |||||
| Vitamin B12 Metabolism | |||||
| Selenium Micronutrient Network | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

