Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08PMB
|
||||
| Former ID |
DIB006278
|
||||
| Drug Name |
Imeglimin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 2 | [524449] | ||
| Company |
Merck KGaA
|
||||
| Structure |
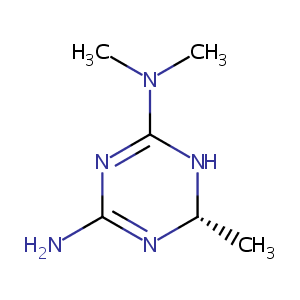
|
Download2D MOL |
|||
| Formula |
C6H13N5
|
||||
| Canonical SMILES |
N1=C(N[C@@H](N=C1N)C)N(C)C
|
||||
| CAS Number |
CAS 775351-65-0
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | AMP-activated protein kinase | Target Info | Modulator | [549117] | |
| KEGG Pathway | FoxO signaling pathway | ||||
| Regulation of autophagy | |||||
| mTOR signaling pathway | |||||
| PI3K-Akt signaling pathway | |||||
| AMPK signaling pathway | |||||
| Circadian rhythm | |||||
| Insulin signaling pathway | |||||
| Adipocytokine signaling pathway | |||||
| Oxytocin signaling pathway | |||||
| Glucagon signaling pathway | |||||
| Non-alcoholic fatty liver disease (NAFLD) | |||||
| Hypertrophic cardiomyopathy (HCM) | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

