Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0A9MR
|
||||
| Former ID |
DAP001208
|
||||
| Drug Name |
Norgestrel
|
||||
| Synonyms |
Capronor; DNorgestrel; Follistrel; Jadelle; Levlen; Levonelle; Levonorgestrel; Levonorgestrelum; Levonova; Methylnorethindrone; Microgyn; Microlut; Microlution; Microluton; Microval; Mirena; Monovar; NORPLANT; Neogest; NorLevo; Nordet; Norgeston; Norgestrelum; Ovranette; Ovrette; Postinor; Preven; Tetragynon; Triagynon; Triciclor; Trigoa; Trivora; LD norgestrel [French]; Ld norgestrel; Levlen ED; Levonorgestrel implants; Logynon ED; Microgest ED; Microgynon CD; Norgestrel [Progestins]; Norplant II; Norplant System in Plastic Container; Plan B; Triquilar ED; Microgynon 21; Microgynon 28; Microgynon 30 ED; Minivlar 30; Monofeme 28; Neogynon 21; Nordette 21; Nordette 28; Norplant 2; SH 70850; SH 850; Stediril 30; Trifeme 28; Trinordiol 21; Trinordiol 28; Triphasil 21; Triphasil 28; Wy 3707; Alpha-Norgestrel; Component of Lo/ovral; D-Norgestrel; Dl-Norgestrel; FH 122-A; LO/Ovral; Levonorgestrelum [INN-Latin]; Levora-21; Levora-28; Mirena (TN); Norgestrelum [INN-Latin]; Norplant (TN); Norplant-2; Ovoplex 30-150; Ovral-Lo; Ovrette (TN); Postinor-2; Rigevidon 21+7; SOH-075; Tri-Levlen 21; Wy-3707; Wy-5104; E-Gen-C; Levonorgestrel [USAN:INN:BAN]; D(-)-Norgestrel; Levonorgestrel (JAN/USP/INN); Norgestrel (JP15/USP/INN); Norgestrel [USAN:BAN:INN:JAN]; Norgestrel [USAN:INN:BAN:JAN]; D-(-)-Norgestrel; Levonelle, D-Norgestrel, Levonova, Levonorgestrel; Norgestrel-(-)-D; Dl-13-beta-Ethyl-17-alpha-ethynyl-19-nortestosterone; (-)-Norgestrel; 13-BETA-ETHYL-17-ALPHA-ETHYNYL-17-BETA-HYDROXYGON-4-EN-3-ONE; 13-Ehyl-17alpha-ethynyl-17-hydroxygon-4-en-3-one; 13-Ethyl-17-alpha-ethynyl-17-beta-hydroxy-4-gonen-3-one; 13-Ethyl-17-alpha-ethynylgon-4-en-17-beta-ol-3-one; 13-Ethyl-17alpha-ethynylgon-4-en-17beta-ol-3-one; 13-beta-Ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one; 13beta-Ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one; 17-Ethynyl-18-methyl-19-nortestosterone; 17-alpha-Ethynyl-13-ethyl-19-nortestosterone; 17alpha-Ethynyl-13-ethyl-19-nortestosterone; 17alpha-Ethynyl-13beta-ethyl-3-oxo-4-estren-17beta-ol; 17alpha-Ethynyl-17-hydroxy-18-methylestr-4-en-3-one; 17alpha-Ethynyl-18-homo-19-nor-testosterone; 17alpha-Ethynyl-18-homo-19-nortestosterone; 17alpha-ethynyl-17beta-hydroxy-18a-homoestr-4-en-3-one; 18,19-Dinor-4-pregnen-20-yn-3-one; 18-Methyl-17-alpha-ethynyl-19-nortestosterone; 18-Methylnorethisterone; 72-HOURS
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Contraceptive Agents
|
||||
| Structure |
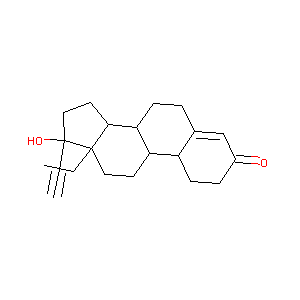
|
Download2D MOL |
|||
| Formula |
C21H28O2
|
||||
| Canonical SMILES |
CCC12CCC3C(C1CCC2(C#C)O)CCC4=CC(=O)CCC34
|
||||
| InChI |
1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
|
||||
| InChIKey |
WWYNJERNGUHSAO-XUDSTZEESA-N
|
||||
| CAS Number |
CAS 6533-00-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10349, 10353, 7848013, 7848017, 7889451, 8159864, 10321210, 11466801, 11467921, 11486521, 11532896, 14801049, 14923455, 24702331, 24897561, 26719646, 29215264, 29281281, 46386677, 46508082, 46509177, 47275737, 47499732, 48094724, 48318626, 49698635, 49963134, 49963135, 50109848, 53787534, 53789027, 56352919, 56422866, 57654445, 71821473, 76968564, 81093159, 85787473, 91147155, 92125812, 92308478, 92309282, 93167212, 103469384, 103913690, 104253340, 104330879, 117622363, 121363483, 124658858
|
||||
| ChEBI ID |
ChEBI:7630
|
||||
| SuperDrug ATC ID |
G03AC03; G03AD01
|
||||
| SuperDrug CAS ID |
cas=000797637
|
||||
| Target and Pathway | |||||
| Target(s) | Progesterone receptor | Target Info | Agonist | [536668] | |
| KEGG Pathway | Oocyte meiosis | ||||
| Progesterone-mediated oocyte maturation | |||||
| Pathway Interaction Database | Cellular roles of Anthrax toxin | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

