Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0B3UJ
|
||||
| Former ID |
DIB004838
|
||||
| Drug Name |
Paliperidone
|
||||
| Synonyms |
Xeplion; Invega Sustenna; Paliperidone palmitate; JNS-010; Paliperidone (depot injection, NanoCrystal); Paliperidone (injectable, NanoCrystal), Elan; Paliperidone (depot injection, NanoCrystal), Johnson & Johnson
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Johnson & Johnson
|
||||
| Structure |
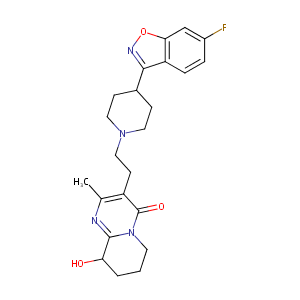
|
Download2D MOL |
|||
| Formula |
C23H27FN4O3
|
||||
| InChI |
InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3
|
||||
| InChIKey |
PMXMIIMHBWHSKN-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 199739-10-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10236700, 14905143, 22395187, 29296762, 46506296, 47207025, 50035150, 51002222, 57339070, 85229944, 85246150, 90341688, 92719038, 92729808, 103604037, 103910909, 104105311, 104397925, 118046559, 121361150, 125307525, 125341102, 126584511, 126621140, 126649064, 126650388, 126667060, 128939139, 131465791, 131549340, 134338126, 135072932, 136367845, 137166111, 142528484, 144116334, 152258953, 152344196, 160647798, 160810375, 160964597, 162185890, 163088563, 163371020, 163564516, 163981461, 164175255, 164785605, 170501956, 171063034
|
||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine receptor | Target Info | Antagonist | [551871] | |
| References | |||||
| Ref 524773 | ClinicalTrials.gov (NCT02146547) European Long-acting Antipsychotics in Schizophrenia Trial. U.S. National Institutes of Health. | ||||
| Ref 542276 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7258). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

