Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0B7EB
|
||||
| Former ID |
DAP000786
|
||||
| Drug Name |
Etoposide
|
||||
| Synonyms |
Eposide; Eposin; Etopol; Etoposido; Etoposidum; Etosid; Lastet; Toposar; VePesid; Vepeside; Zuyeyidal; Demethyl EpipodophyllotoxinEthylidine Glucoside; Vepesid J; E0675; NK 171; VP 16; VP 16213; Demethyl-epiodophyllotoxin ethylidene glucoside; Epipodophyllotoxin VP-16213; Eposin (TN); Etopophos (TN); Etopophos (phosphate salt);Etoposide (VP16); Etoposido [INN-Spanish]; Etoposidum [INN-Latin]; Trans-Etoposide; VP 16 (pharmaceutical); VP 16-213; VP-16; VePESID (TN); Vepesid (TN); DEMETHY-EPIPODOPHYLLOTOXIN, ETHYLIDENE GLUCOSIDE; VP-16 (TN); VP-16-213; Demethylepipodophyllotoxin-beta-D-ethylideneglucoside; Etoposide (JP15/USP/INN); Etoposide [USAN:INN:BAN:JAN]; Eposin, Vepesid, VP-16, Toposar, Etoposide; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI); Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-.beta.-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside); Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-.beta.-D-glucopyranoside); (-)-Etoposide; 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside; 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin ethylidene-.beta.-D-glucoside; 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin; 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside; 4-Demethylepipodophyllotoxin-.beta.-D-ethylideneglucoside
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Bristol Myers Squibb Co Pharmaceutical Research Institute
|
||||
| Structure |
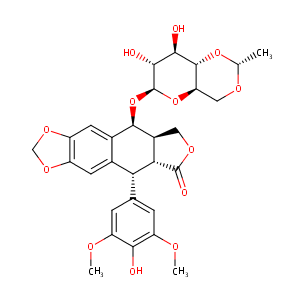
|
Download2D MOL |
|||
| Formula |
C29H32O13
|
||||
| InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
|
||||
| InChIKey |
VJJPUSNTGOMMGY-MRVIYFEKSA-N
|
||||
| CAS Number |
CAS 33419-42-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
4733, 597749, 7847193, 7979208, 8145856, 8174504, 11110521, 12013288, 14886949, 14935915, 24278178, 24769897, 34677904, 46386954, 46500626, 46505434, 47362874, 48182835, 48332078, 49681767, 49699016, 49855364, 53787668, 56463308, 57311878, 71825014, 75974183, 85148351, 85788790, 89850276, 92308660, 92308790, 99437037, 104019076, 104234176, 104324084, 117664408, 117682510, 119525100, 121363248, 124349593, 124659141, 124757097, 124800231, 124893613, 125163901, 127298729, 127298730, 127298731, 127298732
|
||||
| ChEBI ID |
ChEBI:4911
|
||||
| SuperDrug ATC ID |
L01CB01
|
||||
| SuperDrug CAS ID |
cas=033419420
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | DNA topoisomerase II | Target Info | Modulator | ||
| References | |||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 541897 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6815). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

