Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0C2OR
|
||||
| Former ID |
DIB011124
|
||||
| Drug Name |
MIM-D3
|
||||
| Synonyms |
TrkA agonist (ocular disease/Alzheimer's disease), Mimetogen
|
||||
| Indication | Alzheimer disease [ICD9: 331; ICD10:G30] | Phase 3 | [524465] | ||
| Company |
Mimetogen Pharmaceuticals Inc
|
||||
| Structure |
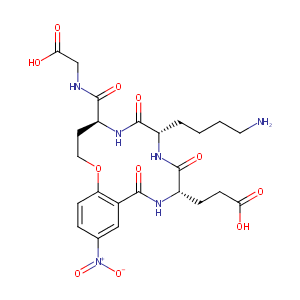
|
Download2D MOL |
|||
| Canonical SMILES |
c12c(ccc(c1)[N+](=O)[O-])OCC[C@H](NC(=O)[C@@H](NC(=O)[C<br />@@H](NC2=O)CCC(=O)O)CCCCN)C(=O)NCC(=O)O
|
||||
| Target and Pathway | |||||
| Target(s) | Neurotrophic tyrosine kinase receptor 1 | Target Info | Agonist | [544321] | |
| NetPath Pathway | IL2 Signaling Pathway | ||||
| Pathway Interaction Database | Neurotrophic factor-mediated Trk receptor signaling | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

