Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0C7JF
|
||||
| Former ID |
DAP000624
|
||||
| Drug Name |
Testolactone
|
||||
| Synonyms |
Fludestrin; Teolit; Teslac; Teslak; Testolacton; Testolactona; Testolactonum; Testolattone; Bristol Myers SquibbBrand of Testolactone; Testolattone [DCIT]; SQ 9538; Bristol-Myers Squibb Brand of Testolactone; DELTA1-Dehydrotestolactone; DELTA1-Dehydrotestololactone; DELTA1-Testololactone; SQ-9538; TESLAC (TN);Teslac (TN); Testolactona [INN-Spanish]; Testolactone [USAN:INN]; Testolactonum [INN-Latin]; Delta(1)-Dehydrotestolactone; Delta(1)-Testolactone; Delta(1)-Testololactone; Delta(1)-testololactone; Delta-1-testololactone; Testolactone (USP/INN); D-Homo-17A-oxaandrosta-1,4-diene-3,17-dione; D-homo-17a-oxaandrosta-1,4-diene-3,17-dione; (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-decahydro-2H-naphtho[2,1-f]chromene-2,8(4bH)-dione; (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-4,4a,4b,5,6,10b,11,12-octahydro-3H-naphtho[2,1-f]chromene-2,8-dione; 1 Dehydrotestolactone; 1,2,3,4,4a,4b,7,9,10,10a-Decahydro-2-hydroxy-2,4b-dimethyl-7-oxo-1-phenanthrenepropionic acid delta-lactone; 1,2-Dehydrotestololactone; 1,2-Didehydrotestololactone; 1,2-didehydrotestololactone; 1-Dehydrotestolactone; 1-Dehydrotestololactone; 1-dehydrotestololactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, .delta.-lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone (8CI); 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13.alpha.-hydroxy-3-oxo-, .delta.-lactone; 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid .delta.-lactone; 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone; 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone; 17a-Oxa-D-homoandrosta-1,4-diene-3,17-dione; 17alpha-Oxo-D-homo-1,4-androstadiene-3,17-dione; 2H-Phenanthro[2,1-b]pyran-2,8(4bH)-dione, 3,4,4a,5,6,10a,10b,11,12,12a-decahydro-10a,12a-dimethyl-, lactone; 3-oxo-13,17-secoandrosta-1,4-dieno-17,13alpha-lactone
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
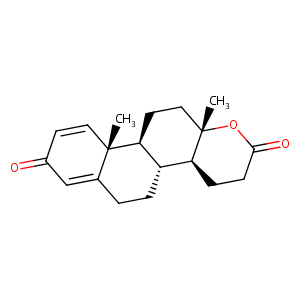
|
Download2D MOL |
|||
| Formula |
C19H24O3
|
||||
| InChI |
InChI=1S/C19H24O3/c1-18-9-7-13(20)11-12(18)3-4-14-15(18)8-10-19(2)16(14)5-6-17(21)22-19/h7,9,11,14-16H,3-6,8,10H2,1-2H3/t14-,15+,16+,18+,19+/m1/s1
|
||||
| InChIKey |
BPEWUONYVDABNZ-DZBHQSCQSA-N
|
||||
| CAS Number |
CAS 968-93-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
5265, 85554, 7847221, 8148207, 8160380, 11406301, 12208826, 16241793, 29281886, 46508076, 48416603, 50003964, 53788634, 57328321, 77336000, 85300778, 103589303, 104332677, 126690566, 134337914, 134980940, 136309516, 136349969, 137005559, 137545235, 144205129, 151979558, 160846950, 160964233, 175265391, 178103876, 179316044, 184545981, 223402669, 226396123, 241169804, 251884479, 252357062
|
||||
| ChEBI ID |
ChEBI:9460
|
||||
| Target and Pathway | |||||
| Target(s) | Cytochrome P450 19 | Target Info | Inhibitor | [535343], [538089] | |
| NetPath Pathway | FSH Signaling Pathway | ||||
| PANTHER Pathway | Androgen/estrogene/progesterone biosynthesis | ||||
| PathWhiz Pathway | Androgen and Estrogen Metabolism | ||||
| Reactome | Endogenous sterols | ||||
| References | |||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 542325 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7303). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

