Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0D1BR
|
||||
| Former ID |
DNCL002989
|
||||
| Drug Name |
GS-7340
|
||||
| Synonyms |
NtRTI
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Human immunodeficiency virus infection [ICD9: 279.3; ICD10:B20-B26] | Phase 2 | [523854] | ||
| Company |
Gilead Sciences
|
||||
| Structure |
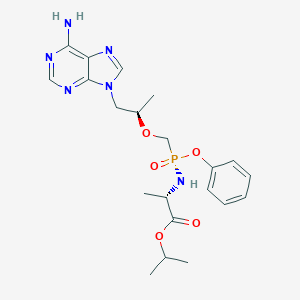
|
Download2D MOL |
|||
| Formula |
C21H29N6O5P
|
||||
| Canonical SMILES |
CC(C)OC(=O)C(C)NP(=O)(COC(C)CN1C=NC2=C1N=CN=C2N)OC3=CC=<br />CC=C3
|
||||
| InChI |
1S/C21H29N6O5P/c1-14(2)31-21(28)16(4)26-33(29,32-17-8-6-5-7-9-17)13-30-15(3)10-27-12-25-18-19(22)23-11-24-20(18)27/h5-9,11-12,14-16H,10,13H2,1-4H3,(H,26,29)(H2,22,23,24)/t15-,16+,33-/m1/s1
|
||||
| InChIKey |
LDEKQSIMHVQZJK-GNGHXOLXSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | HIV reverse transcriptase | Target Info | Inhibitor | [527526] | |
| References | |||||
| Ref 523854 | ClinicalTrials.gov (NCT01565850) Safety and Efficacy of Darunavir/Cobicistat/Emtricitabine/GS-7340 Single Tablet Regimen Versus Cobicistat-boosted Darunavir Plus Emtricitabine/Tenofovir Disoproxil Fumarate Fixed Dose Combination in HIV-1 Infected, Antiretroviral Treatment Naive Adults. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

