Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0FM7Z
|
||||
| Former ID |
DIB007181
|
||||
| Drug Name |
BL-1021
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89] | Phase 1 | [523040] | ||
| Company |
Tel Aviv University
|
||||
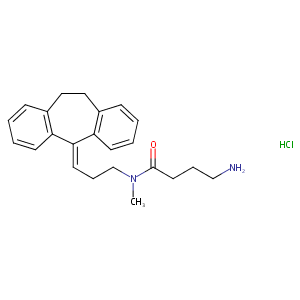
| Structure |
|
Download2D MOL |
|||
| Formula |
C23H29ClN2O
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Sodium-dependent noradrenaline transporter | Target Info | Modulator | ||
| Sodium-dependent serotonin transporter | Target Info | Modulator | |||
| KEGG Pathway | Serotonergic synapse | ||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

