Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0FT4R
|
||||
| Former ID |
DNC001333
|
||||
| Drug Name |
Sivelestat
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
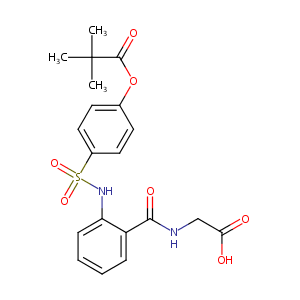
|
Download2D MOL |
|||
| Formula |
C20H22N2O7S
|
||||
| InChI |
InChI=1S/C20H22N2O7S/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24)
|
||||
| InChIKey |
BTGNGJJLZOIYID-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 331731-18-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10234395, 14759000, 17397876, 26758657, 44436677, 50064306, 50998781, 53800789, 57338198, 76715600, 103277098, 103984170, 104379537, 123392712, 125680070, 126682396, 127564958, 134340533, 135065365, 137156953, 137263939, 140115436, 162183567, 163125720, 163688184, 164192385, 164224889, 172821371, 174006339, 178103057, 179149758, 198973808, 204360313, 208264999, 210275598, 210281257, 215775576, 223657139, 226494344, 242501916, 251971107, 252353896, 252356633
|
||||
| Target and Pathway | |||||
| Target(s) | Leukocyte elastase | Target Info | Inhibitor | [537032] | |
| Elastase 1 | Target Info | Inhibitor | [537405] | ||
| Pathway Interaction Database | Urokinase-type plasminogen activator (uPA) and uPAR-mediated signaling | ||||
| C-MYB transcription factor network | |||||
| References | |||||
| Ref 521724 | ClinicalTrials.gov (NCT00219375) Study of Sivelestat Sodium Hydrate in Acute Lung Injury (ALI) Associated With Systemic Inflammatory Response Syndrome (SIRS) in Japan. U.S. National Institutes of Health. | ||||
| Ref 541582 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6441). | ||||
| Ref 537032 | Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NF-kB. Inflamm Res. 2009 Apr;58(4):198-203. | ||||
| Ref 537405 | Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both ARDS and DIC patients. Shock. 2009 May 18. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

