Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0GL7U
|
||||
| Former ID |
DAP001212
|
||||
| Drug Name |
Norethindrone
|
||||
| Synonyms |
Activella; Anhydrohydroxynorprogesterone; Anovulatorio; Anovule; Brevicon; Brevinor; Camila; Ciclovulan; Conceplan;Conludaf; Conludag; Demulen; Errin; Estrinor; Ethinylnortestosterone; Ethynylmortestosterone; Ethynylnortestosterone; Gencept; Genora; Gestest; Jenest; Loestrin; Menzol; Microneth; Micronett; Micronor; Micronovum; Milli; Minovlar; Nelova; Nodiol; Noraethisteronum; Noralutin; Norcept; Norcolut; Norcolute; Noresthisterone; Norethadrone; Norethin; Norethindirone; Norethisteron; Norethisterone; Norethisteronum; Norethyndron; Norethynodron; Norethynodrone; Noretisterona; Noretisterone; Norfor; Norgestin; Noriday; Norluten; Norlutin; Norluton; Normapause; Norpregneninlone; Norpregneninolone; Norpregneninotone; Orlest; Proluteasi; Synphase; Triella; Utovlan; Utovlar; Component of Noriday; Norethindrone Norethisterone; Norethindrone [USAN]; Norethisterone [Progestins]; Noretisterone [DCIT]; Primolut N; Brevinor 21; Brevinor 28; Noriday 28; Ortho 1 35; Ortho 7 7 7; Ovysmen 1 35; SC 4640; Synphasic 28; Trinovum 21; Brevinor-1 21; Brevinor-1 28; Camila (TN); Jenest-28; Micronor (TN); Mini-Pe; Mini-pill; Nor-QD; Nora-BE; Norcept-E; Norethin 1/35 E; Norethin 1/50 M; Norethindrone (USP); Norethisterone (JP15); Norethisteronum [INN-Latin]; Noretisterona [INN-Spanish]; Ortho-Novum 1 35; Ortho-Novum 1 50; Ortho-Novum 7 7 7; Ovysmen 0.5 35; Primolut-N; Tri-Norinyl; Ethinyl-19-nortestosterone; Nor-Q.D; Primolut-N (TN); Nor-Q.D.; 17-Ethinyl-19-nortestosterone; 17-Ethynyl-17-hydroxyestr-4-en-3-one; 17-alpha-Ethynyl-19-nortestosterone; 17-alpha-Ethynyl-4-estren-17-ol-3-one; 17-ethynyl-17beta-hydroxyestr-4-en-3-one; 17.alpha.-Ethinyl-19-nortestosterone; 17.alpha.-Ethynyl-19-nortestosterone; 17.alpha.-Ethynyl-4-estren-17-ol-3-one; 17alpha-Ethinyl-19-nortestosterone; 17alpha-Ethynyl-19-nortestosterone; 17alpha-Ethynyl-4-estren-17-ol-3-one; 19-Nor-17-alpha-ethynyltestosterone; 19-Nor-17-ethinyltestosterone; 19-Nor-17-ethinyltestosterone; 19-Nor-17.alpha.-ethynyltestosterone; 19-Nor-17alpa-ethynyltestosterone; 19-Nor-17alpha-ethynyltestosterone; 19-Nor-ethindrone; 19-Nor-ethinyl-4,5-testosterone; 19-Norethindrone; 19-Norethinyltestosterone; 19-Norethisterone; 4-Estren-17alpha-ethynyl-17beta-ol-3-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Approved | [551871] | ||
| Therapeutic Class |
Contraceptive Agents
|
||||
| Company |
Parke Davis Div Warner Lambert Co
|
||||
| Structure |
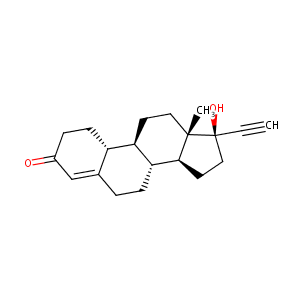
|
Download2D MOL |
|||
| Formula |
C20H26O2
|
||||
| InChI |
InChI=1S/C20H26O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,12,15-18,22H,4-11H2,2H3/t15-,16+,17+,18-,19-,20-/m0/s1
|
||||
| InChIKey |
VIKNJXKGJWUCNN-XGXHKTLJSA-N
|
||||
| CAS Number |
CAS 68-22-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7499, 7847250, 7980169, 8153912, 10321609, 11466281, 11467401, 11486021, 12146103, 14873691, 14873694, 24702322, 24870259, 24897669, 25621785, 29225226, 46504816, 47440543, 47736755, 48185240, 48259494, 48416339, 49647479, 49648521, 49698406, 49998430, 50124391, 50139262, 53788259, 56313695, 57581168, 57650825, 77234545, 85788451, 87573784, 92125032, 92309077, 92710502, 103338617, 103914098, 104036985, 104170074, 104311472, 115354325, 121362406, 121362703, 124799740, 124893626, 126603566, 126631815
|
||||
| ChEBI ID |
ChEBI:7627
|
||||
| SuperDrug ATC ID |
G03AC01; G03DC02
|
||||
| SuperDrug CAS ID |
cas=000068224
|
||||
| Target and Pathway | |||||
| Target(s) | Progesterone receptor | Target Info | Agonist | [535980] | |
| KEGG Pathway | Oocyte meiosis | ||||
| Progesterone-mediated oocyte maturation | |||||
| Pathway Interaction Database | Cellular roles of Anthrax toxin | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

