Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H9DA
|
||||
| Former ID |
DIB003378
|
||||
| Drug Name |
AZD6738
|
||||
| Indication | Chronic lymphocytic leukaemia [ICD10:C91] | Phase 1 | [524454] | ||
| Company |
Astrazeneca; eagle pharmaceuticals
|
||||
| Structure |
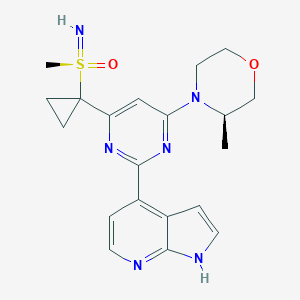
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Serine threonine protein kinase ATR | Target Info | Inhibitor | [532880], [532980], [550504] | |
| PANTHER Pathway | p53 pathway | ||||
| p53 pathway feedback loops 2 | |||||
| Pathway Interaction Database | Fanconi anemia pathway | ||||
| ATR signaling pathway | |||||
| Signaling events mediated by TCPTP | |||||
| Circadian rhythm pathway | |||||
| BARD1 signaling events | |||||
| p53 pathway | |||||
| Reactome | Meiotic synapsis | ||||
| Activation of ATR in response to replication stress | |||||
| Regulation of HSF1-mediated heat shock response | |||||
| HDR through Single Strand Annealing (SSA) | |||||
| Processing of DNA double-strand break ends | |||||
| Presynaptic phase of homologous DNA pairing and strand exchange | |||||
| G2/M DNA damage checkpoint | |||||
| References | |||||
| Ref 532880 | Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget. 2014 Jul 30;5(14):5674-85. | ||||
| Ref 532980 | ATR inhibitors VE-821 and VX-970 sensitize cancer cells to topoisomerase i inhibitors by disabling DNA replication initiation and fork elongation responses. Cancer Res. 2014 Dec 1;74(23):6968-79. | ||||
| Ref 550504 | National Cancer Institute Drug Dictionary (drug id 754022). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

