Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0I3YX
|
||||
| Former ID |
DNCL001889
|
||||
| Drug Name |
Capadenoson
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Atrial fibrillation [ICD9: 272, 427.31; ICD10:E78, I48] | Phase 2 | [522175] | ||
| Company |
Bayer HealthCare Pharmaceuticals
|
||||
| Structure |
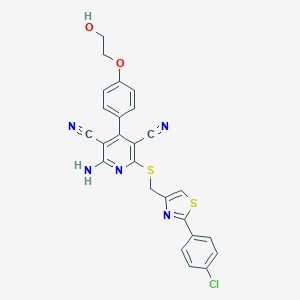
|
Download2D MOL |
|||
| Formula |
C25H18ClN5O2S2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Adenosine A1 receptor | Target Info | Agonist | [529810] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| RANKL Signaling Pathway | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

