Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0IV7Z
|
||||
| Former ID |
DIB004447
|
||||
| Drug Name |
AZD-8418
|
||||
| Indication | Schizophrenia [ICD9: 295; ICD10:F20] | Phase 1 | [522881] | ||
| Company |
AstraZeneca plc
|
||||
| Structure |
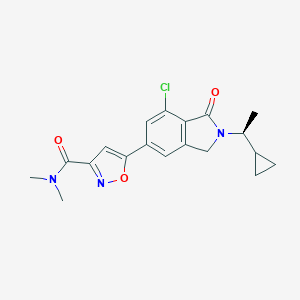
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Glutamate receptor AMPA subtype | Target Info | Modulator | [549032] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

