Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0K7WK
|
||||
| Former ID |
DAP000221
|
||||
| Drug Name |
Ondansetron
|
||||
| Synonyms |
DESMETHYLONDANSETRON; Zofran; Zophren; Zudan; Sandoz ondansetron; ZOFRAN IN PLASTIC CONTAINER; Zofran ODT; GR 38032; GR 38032X; GR38032F; Apo-ondansetron; GR-38032F; Novo-ondansetron; Ondansetron (Zofran); PHL-ondansetron; PMS-ondansetron; Ratio-ondansetron; SN-307; Zofran (TN); Zofran ODT (TN); Ondansetron [USAN:INN:BAN]; Ondansetron (JAN/USP/INN); Ondansetron, (+,-)-Isomer; (RS)-1,2,3,9-Tetrahydro-9-methyl-3-(2-methylimidazol-1-ylmethyl)carbazol-4-one; 1,2,3,9-Tetrahydro-9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-4H-carbazol-4-one; 9-Methyl-3-(2-methyl-imidazol-1-ylmethyl)-1,2,3,9-tetrahydro-carbazol-4-one; 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one; 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiemetics
|
||||
| Company |
GlaxoSmithKline
|
||||
| Structure |
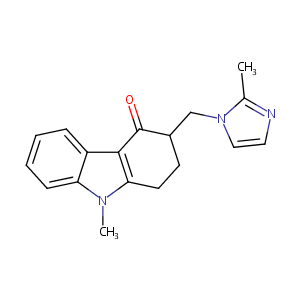
|
Download2D MOL |
|||
| Formula |
C18H19N3O
|
||||
| InChI |
InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3
|
||||
| InChIKey |
FELGMEQIXOGIFQ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 99614-02-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9533, 3206266, 3966923, 4696243, 7847522, 7980201, 8152820, 11137931, 11427135, 11467086, 11468206, 11486674, 11501532, 14873468, 29223684, 46504819, 47389509, 47686452, 47686453, 47909550, 47909551, 47984334, 48416352, 49698991, 49835876, 49901808, 50064204, 50949003, 53787007, 57322341, 58007430, 85174434, 85209216, 85787808, 85788905, 92309152, 93624973, 103166621, 103915041, 104170204, 104225172, 104306859, 105146690, 121362488, 124579243, 124893606, 125324981, 125360632, 125814185, 126525328
|
||||
| SuperDrug ATC ID |
A04AA01
|
||||
| SuperDrug CAS ID |
cas=099614025
|
||||
| Target and Pathway | |||||
| Target(s) | 5-Hydroxytryptamine 3 receptor | Target Info | Agonist | [538141] | |
| References | |||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 539441 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2290). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

