Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0KV4Z
|
||||
| Former ID |
DIB008978
|
||||
| Drug Name |
CI-949
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Asthma [ICD10:J45] | Discontinued in Phase 2 | [544546] | ||
| Company |
Dura Pharmaceuticals Inc
|
||||
| Structure |
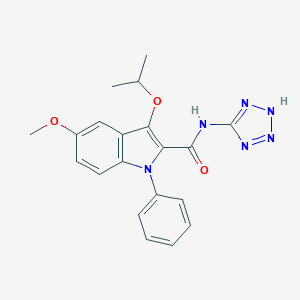
|
Download2D MOL |
|||
| Formula |
C26H34N10O5
|
||||
| Canonical SMILES |
c1(n(c2c(c1OC(C)C)cc(cc2)OC)c1ccccc1)C(=O)Nc1nnn[nH]1.C<br />(=N)(NCCC[C@@H](C(=O)O)N)N
|
||||
| CAS Number |
CAS 121530-58-3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Leukotriene B4 receptor 1 | Target Info | Modulator | [528462] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| NetPath Pathway | IL4 Signaling Pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

