Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0L6LD
|
||||
| Former ID |
DIB012379
|
||||
| Drug Name |
AZD-3199
|
||||
| Indication | Asthma [ICD10:J45] | Phase 2 | [522407] | ||
| Structure |
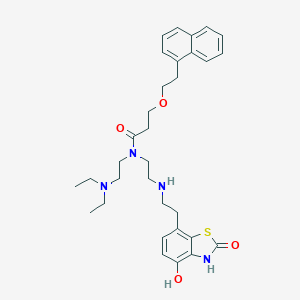
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Beta-2 adrenergic receptor | Target Info | Agonist | [544464] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | ||||
| Arf6 signaling events | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

