Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O1TC
|
||||
| Former ID |
DAP000806
|
||||
| Drug Name |
Gamma-Homolinolenic acid
|
||||
| Synonyms |
DGLA; BML3-B11; Ro 12-1989; Star GLA (GNC); Bishomo-gamma-linolenic acid; C 20:3 n-6; Dihomo-gamma-linolenic acid; Homo-gamma-linolenic acid; Homo-gamma-linolensaeure; Tona-lean 1000 CLA (Action Labs); Cis-8,11,14-Eicosatrienoic Acid; Eicosa-8Z,11Z,14Z-trienoic acid; All-cis-8,11,14-Eicosatrienoic acid; All-cis-8,11,14-icosatrienoic acid; All-cis-Eicosa-8,11,14-triensaeure; Cis,cis,cis-8,11,14-Eicosatrienoic acid; Cis-8,cis-11,cis-14-Eicosatrienoic acid; (8E,11E,14E)-8,11,14-Icosatrienoic acid; (8Z,11Z,14Z)-Icosatrienoic acid; (8Z,11Z,14Z)-icosa-8,11,14-trienoic acid; (Z,Z,Z)-8,11,14-Eicosatrienoate; (Z,Z,Z)-8,11,14-Eicosatrienoic acid; (Z,Z,Z)-8,11,14-Icosatrienoate; (Z,Z,Z)-8,11,14-Icosatrienoicacid; 20:3, n-6,9,12 all-cis; 8, 11, 14-eicosatrienoic acid; 8,11,14-Eicosatrienoate; 8,11,14-Eicosatrienoic acid; 8,11,14-Eicosatrienoic acid, (8Z,11Z,14Z)-(9CI); 8,11,14-Eicosatrienoic acid, (Z,Z,Z)-(8CI); 8,11,14-Icosatrienoate; 8,11,14-all-cis-Eicosatrienoic acid; 8c,11c,14c-Eicosatriensaeure; 8c,11c,14c-eicosatrienoic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Dietary shortage [ICD9: 260-269; ICD10:E40-E46] | Approved | [537917] | ||
| Therapeutic Class |
Dietary supplement
|
||||
| Structure |
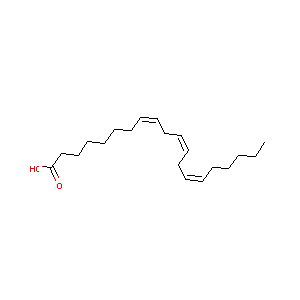
|
Download2D MOL |
|||
| Formula |
C20H34O2
|
||||
| Canonical SMILES |
CCCCCC=CCC=CCC=CCCCCCCC(=O)O
|
||||
| InChI |
1S/C20H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13H,2-5,8,11,14-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-
|
||||
| InChIKey |
HOBAELRKJCKHQD-QNEBEIHSSA-N
|
||||
| CAS Number |
CAS 1783-84-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
6112, 841844, 7850023, 7888613, 8616330, 14849827, 24894557, 26754937, 26754938, 39289665, 46508866, 47364932, 47885171, 50110840, 50834734, 57357798, 85787438, 87322643, 92309702, 99300616, 99302134, 103636894, 104046532, 104115205, 113853558, 126524681, 134222871, 134338087, 134978911, 137205464, 139646151, 160963503, 179150079, 184568160, 198986692, 225022227, 227115049, 250134279, 252368023, 252455426, 252457617
|
||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin G/H synthase 1 | Target Info | Inhibitor | [535090], [535222], [535424] | |
| BioCyc Pathway | C20 prostanoid biosynthesis | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| References | |||||
| Ref 535090 | Mutational and X-ray crystallographic analysis of the interaction of dihomo-gamma -linolenic acid with prostaglandin endoperoxide H synthases. J Biol Chem. 2001 Mar 30;276(13):10358-65. Epub 2000 Dec 19. | ||||
| Ref 535222 | Structure of eicosapentaenoic and linoleic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J Biol Chem. 2001 Oct 5;276(40):37547-55. Epub 2001 Jul 27. | ||||
| Ref 535424 | Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002 Jul 15;365(Pt 2):489-96. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

