Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0QD1G
|
||||
| Former ID |
DNC000600
|
||||
| Drug Name |
Elvitegravir
|
||||
| Synonyms |
EVG
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Human immunodeficiency virus infection [ICD9: 279.3; ICD10:B20-B26] | Approved | [532651] | ||
| Structure |
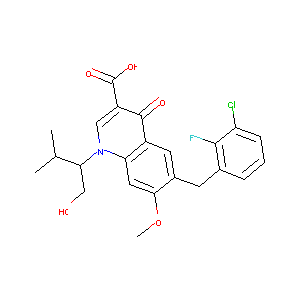
|
Download2D MOL |
|||
| Formula |
C23H23ClFNO5
|
||||
| Canonical SMILES |
CC(C)C(CO)N1C=C(C(=O)C2=CC(=C(C=C21)OC)CC3=C(C(=CC=C3)C<br />l)F)C(=O)O
|
||||
| InChI |
1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1
|
||||
| InChIKey |
JUZYLCPPVHEVSV-LJQANCHMSA-N
|
||||
| CAS Number |
CAS 697761-98-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7843482, 8615111, 12015964, 16816905, 39286528, 57357690, 78878548, 87457715, 87457725, 99436935, 103481427, 104067806, 113844313, 124757574, 125164378, 134964404, 135269865, 136348707, 136367562, 136367953, 137006544, 139530791, 143498895, 144115938, 152237703, 152258597, 152344346, 160647432, 160962968, 162011862, 162037756, 162201766, 164198365, 172096660, 174007151, 174530418, 175266793, 176484884, 177748762, 180387581, 188899508, 198992862, 208265476, 223400590, 223705173, 223723295, 224244595, 227014509, 247090214, 249814482
|
||||
| SuperDrug ATC ID |
J05AX11
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | HIV integrase | Target Info | Modulator | [532651] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

