Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0R8ER
|
||||
| Former ID |
DAP001000
|
||||
| Drug Name |
Enoxacin
|
||||
| Synonyms |
Almitil; Bactidan; Comprecin; Enoram; Enoxacine; Enoxacino; Enoxacinum; Enoxin; Enoxor; Flumark; Penetrex; Enoxacin Sesquihydrate; Enoxacine [French]; Enoxacino [Spanish]; Enoxacinum [Latin]; Faulding Brand of Enoxacin; Pierre Fabre Brand of Enoxacin Sesquihydrate; Rhone Poulenc Rorer Brand of Enoxacin Sesquihydrate; AT 2266; AT2266; CI919; CL23362; E0762; PD 107779; PD107779; AT-2266; Almitil (TN); Bactidan (TN); Bactidron (TN); Comprecin (TN); Enoksetin (TN); Enoxen (TN); Enoxin (TN); Enoxor (TN); Enroxil (TN); Flumark (TN); Gyramid (TN); PD-107779; Penetrex (TN); Rhone-Poulenc Rorer Brand of Enoxacin Sesquihydrate; Sesquihydrate, Enoxacin; Vinone (TN); Enoxacin (USAN/INN); Enoxacin [USAN:BAN:INN:JAN]; 1,8-Naphthyridine-3-carboxylic acid, 6-fluoro-1,4-dihydro-4-oxo-7-piperazinyl; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine-3-carboxylic acid; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-piperazinyl]-1,8-naphthyridine-3-carboxylic acid; 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,8-naphthyridine-3-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiinfective Agents
|
||||
| Structure |
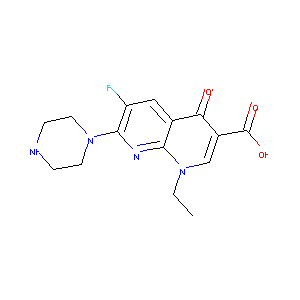
|
Download2D MOL |
|||
| Formula |
C15H17FN4O3
|
||||
| Canonical SMILES |
CCN1C=C(C(=O)C2=CC(=C(N=C21)N3CCNCC3)F)C(=O)O
|
||||
| InChI |
1S/C15H17FN4O3/c1-2-19-8-10(15(22)23)12(21)9-7-11(16)14(18-13(9)19)20-5-3-17-4-6-20/h7-8,17H,2-6H2,1H3,(H,22,23)
|
||||
| InChIKey |
IDYZIJYBMGIQMJ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 74011-58-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9193, 494643, 598045, 855964, 7656850, 7847376, 7979166, 8149934, 8152051, 10321663, 11112842, 11335595, 11360834, 11364433, 11366995, 11369557, 11372679, 11373790, 11377719, 11414007, 11461806, 11466381, 11467501, 11485538, 11486102, 11489547, 11491385, 11491981, 11495353, 12012631, 14899277, 24894528, 26612426, 26680210, 26747059, 26747060, 29222370, 46507505, 47216718, 47365122, 47365123, 47440190, 47736413, 47885347, 48110393, 48110394, 48184940, 48334424, 48415943, 49698449
|
||||
| SuperDrug ATC ID |
J01MA04
|
||||
| SuperDrug CAS ID |
cas=074011588
|
||||
| Target and Pathway | |||||
| Target(s) | DNA topoisomerase II | Target Info | Inhibitor | [537741] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

