Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0S7UU
|
||||
| Former ID |
DIB000384
|
||||
| Drug Name |
PF-06260414
|
||||
| Indication | Cachexia [ICD9: 799.4; ICD10:R64] | Discontinued in Phase 1 | [549537] | ||
| Structure |
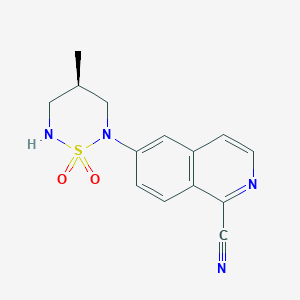
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Androgen receptor | Target Info | Modulator | [550356] | |
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | ||||
| Coregulation of Androgen receptor activity | |||||
| Regulation of Androgen receptor activity | |||||
| Nongenotropic Androgen signaling | |||||
| Regulation of nuclear beta catenin signaling and target gene transcription | |||||
| FOXA1 transcription factor network | |||||
| Notch-mediated HES/HEY network | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

