Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T7QF
|
||||
| Former ID |
DNC006242
|
||||
| Drug Name |
(S)-tert-butyl 1-oxohexan-2-ylcarbamate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [527872] | ||
| Structure |
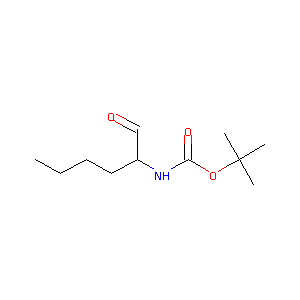
|
Download2D MOL |
|||
| Formula |
C11H21NO3
|
||||
| Canonical SMILES |
CCCCC(C=O)NC(=O)OC(C)(C)C
|
||||
| InChI |
1S/C11H21NO3/c1-5-6-7-9(8-13)12-10(14)15-11(2,3)4/h8-9H,5-7H2,1-4H3,(H,12,14)/t9-/m0/s1
|
||||
| InChIKey |
OBMGXPJNZKYOQY-VIFPVBQESA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cathepsin L | Target Info | Inhibitor | [527872] | |
| Cathepsin K | Target Info | Inhibitor | [527872] | ||
| Reactome | Endosomal/Vacuolar pathway | ||||
| Collagen degradation | |||||
| Degradation of the extracellular matrix | |||||
| Trafficking and processing of endosomal TLR | |||||
| Assembly of collagen fibrils and other multimeric structures | |||||
| MHC class II antigen presentationR-HSA-1442490:Collagen degradation | |||||
| Activation of Matrix Metalloproteinases | |||||
| MHC class II antigen presentation | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

